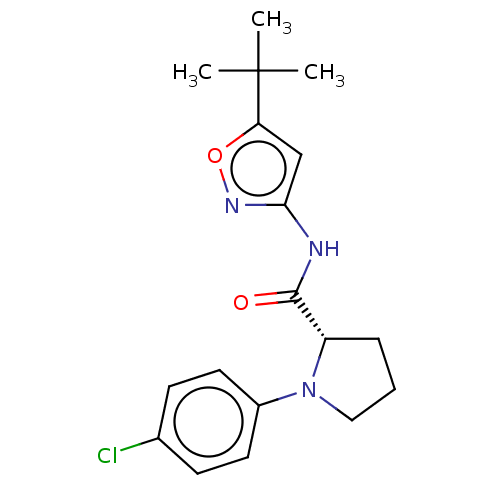
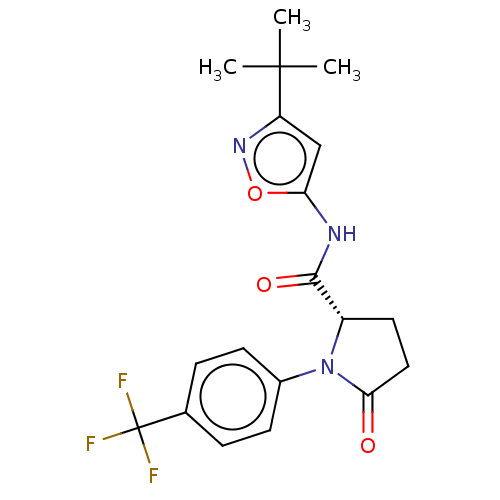
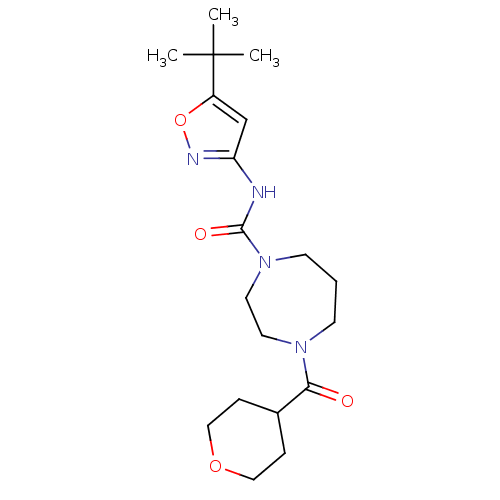
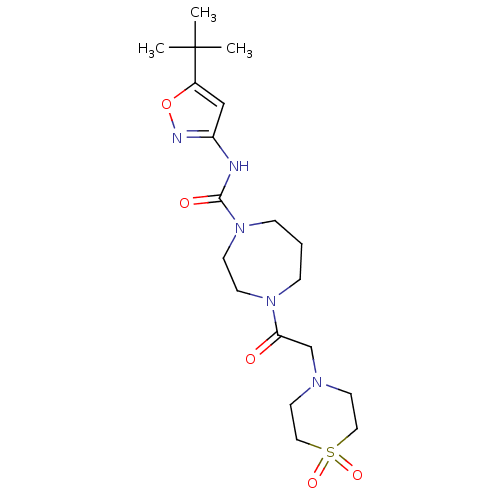
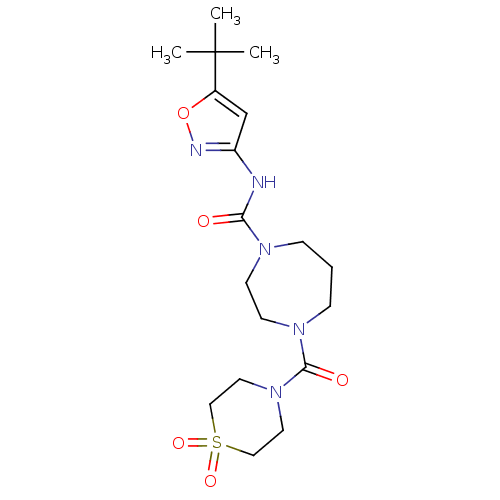
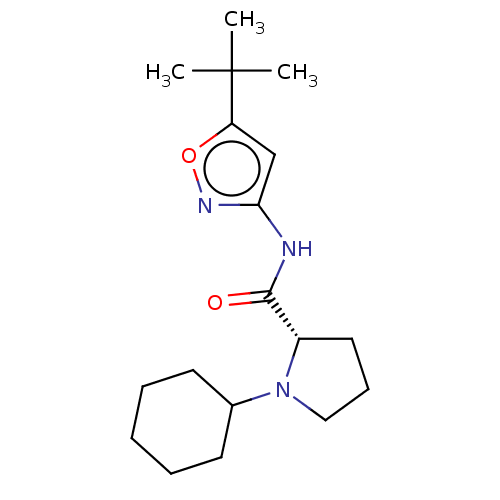
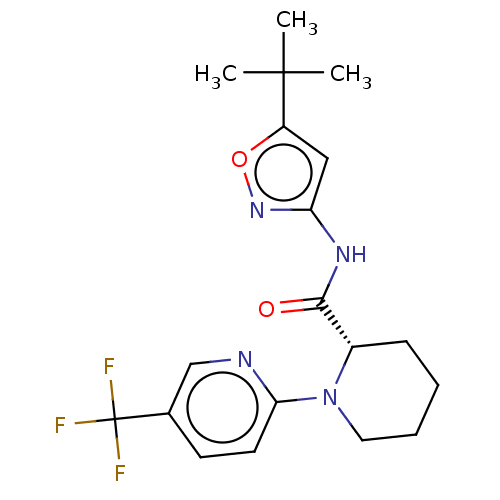
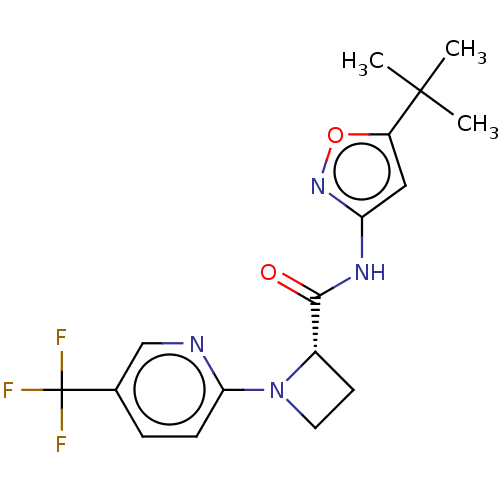
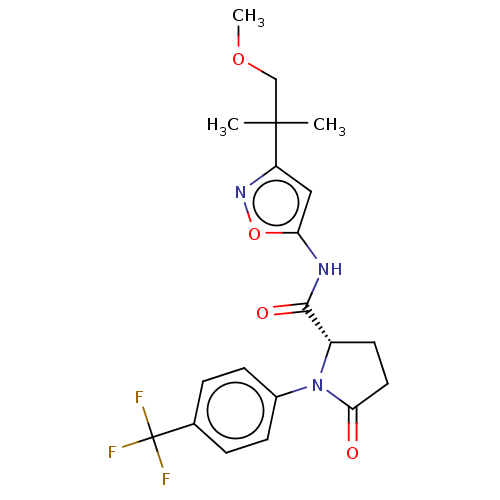
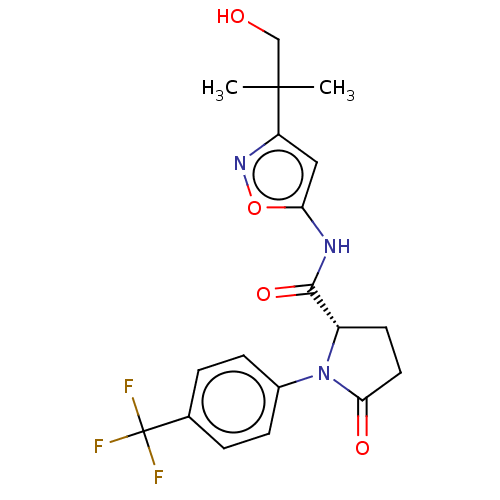
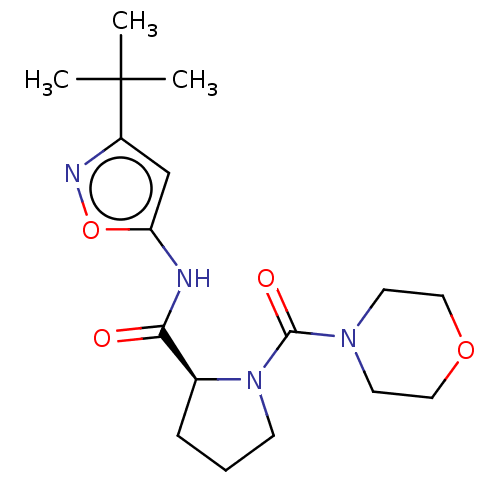
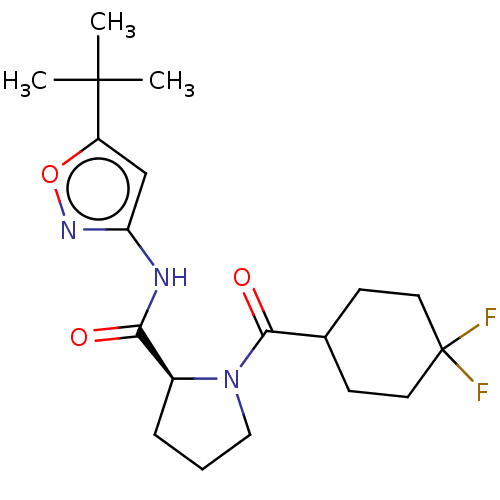
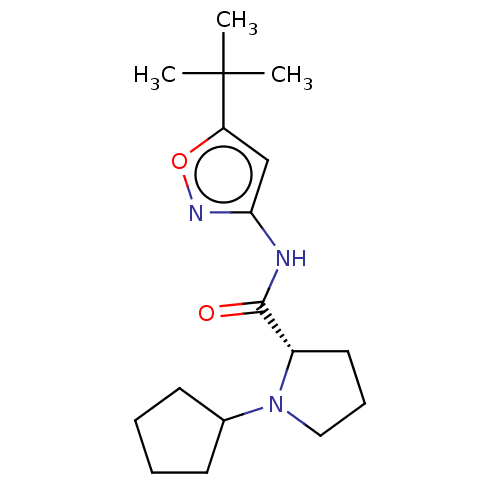
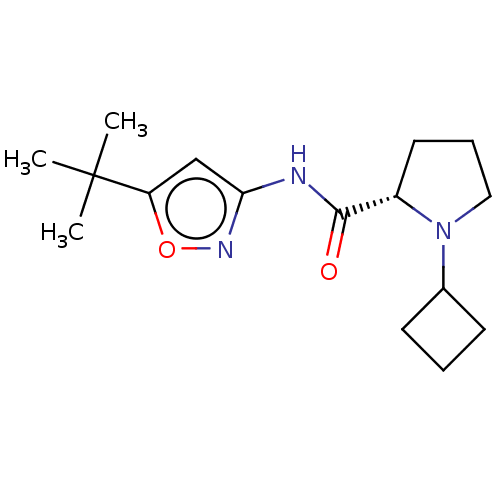
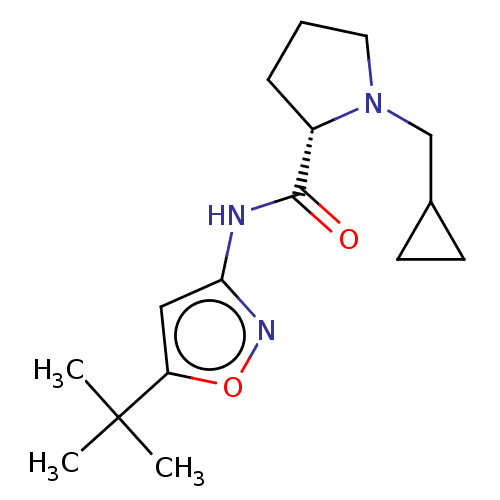
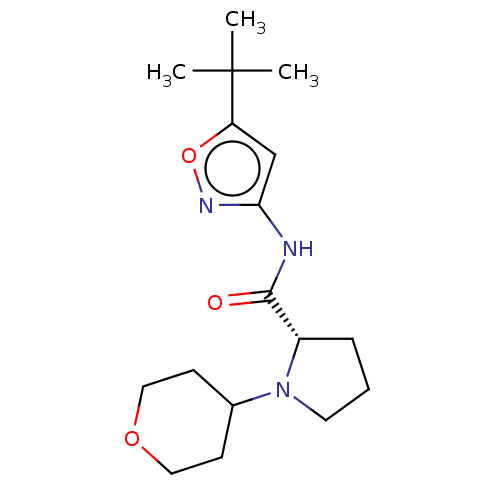
Affinity DataKi: 3nMAssay Description:Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK cellsMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 630nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarinMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarinMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarinMore data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarinMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 using flourescent probe 7-benzyloxyquinoline and 7-benzyloxy-4-(trifluoromethyl)-coumarin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 using flourescent probe 7-methoxy-4-trifluoromethylcoumarinMore data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 flourescent probe 3-[2-(N,N-diethyl-N-methylamino)ethyl]-7-methoxy-4-methylcoumarinMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 4.60E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Homo sapiens (Human))
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.190nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.230nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 30nMAssay Description:Displacement of [3H]-CP-55,940 from human CB1 receptor expressed in HEK293 cell membranes cells by SPAMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Displacement of [3H]-WIN-55,212-2 from human CB2 receptor expressed in HEK293 cell membranes cells by SPAMore data for this Ligand-Target Pair
Affinity DataEC50: 250nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 53nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+3nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0930nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0700nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.20nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 38nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 4.00E+3nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 390nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.20E+3nMAssay Description:Agonist activity at human CB2 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 307nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+3nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 390nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 2.40E+3nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 9.80E+4nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.01E+4nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 4.10E+4nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 4.90E+3nMAssay Description:Agonist activity at human CB1 receptor expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP production after 30 minsMore data for this Ligand-Target Pair