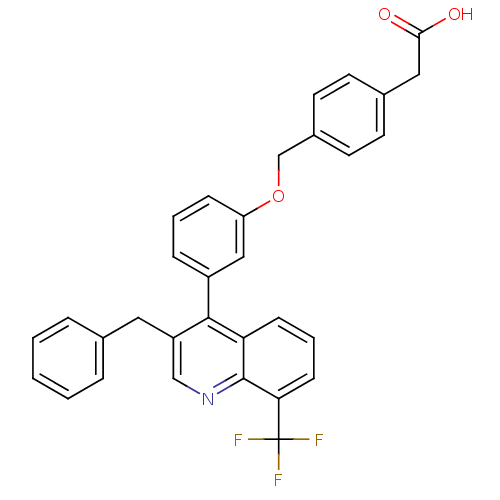
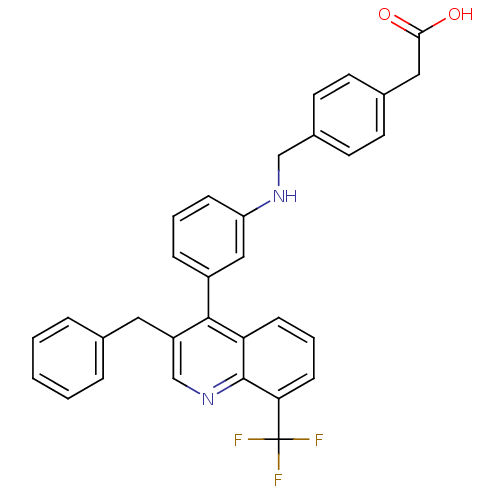
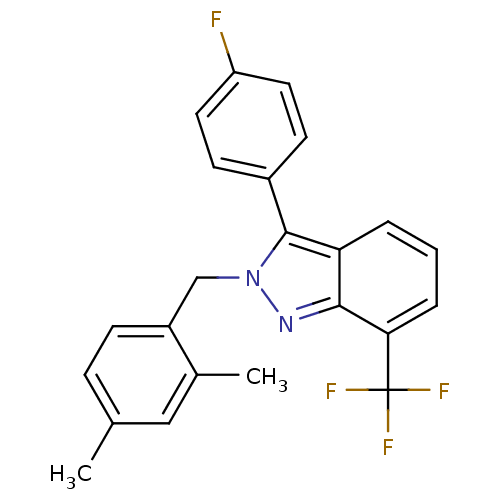
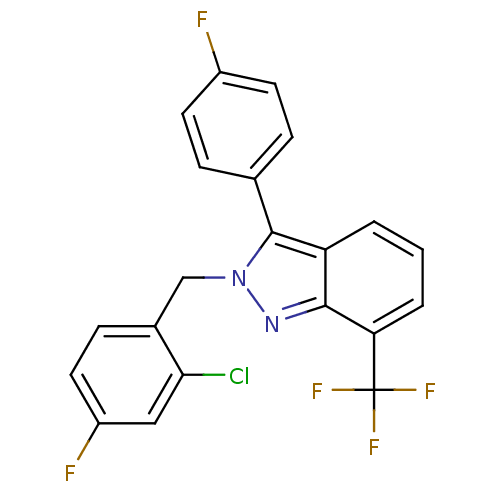
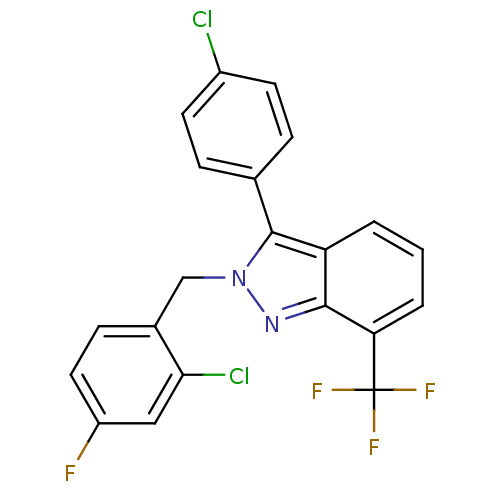
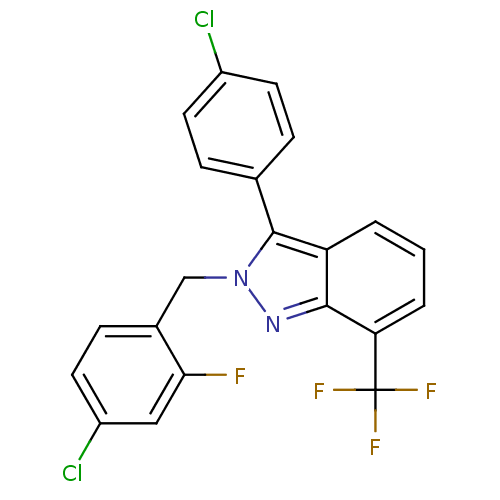
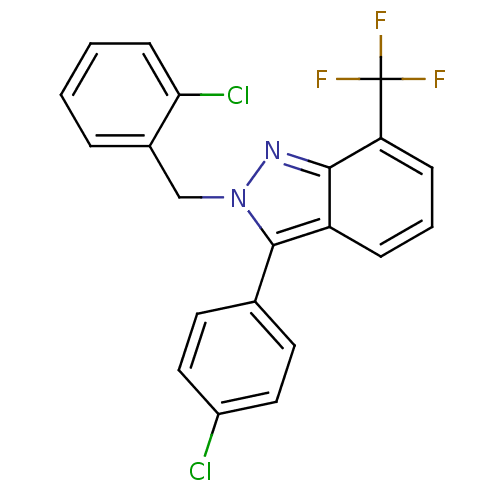
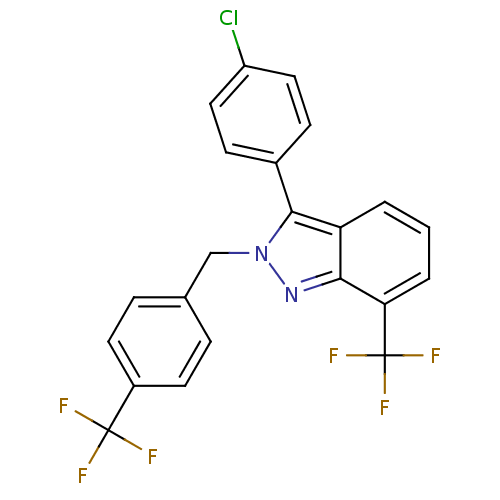
Affinity DataIC50: 2nM EC50: 90nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 2nM EC50: 90nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 8nM EC50: 160nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 170nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q2XW4H4GPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q2XW4H4GPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 10nM EC50: 990nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 10nM EC50: 240nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 310nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 13nM EC50: 140nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 24nM EC50: 3.67E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 30nM EC50: 1.12E+4nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 32nM EC50: 2.89E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 33nM EC50: 4.15E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 38nM EC50: 7.44E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 38nM EC50: 9.80E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 40nM EC50: 1.02E+4nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 41nM EC50: 3.26E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 48nM EC50: 1.55E+4nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 49nM EC50: 5.72E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 58nM EC50: 9.05E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 78nM EC50: 1.81E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 100nM EC50: 660nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 179nM EC50: 6.66E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 248nM EC50: 6.10E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 252nM EC50: 1.44E+4nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 260nM EC50: 1.33E+4nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 279nM EC50: 4.27E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 297nM EC50: 5.02E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 304nM EC50: 1.34E+4nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 317nM EC50: 8.40E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 379nM EC50: 1.52E+4nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 401nM EC50: 1.28E+4nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 465nM EC50: 1.58E+4nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+3nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair