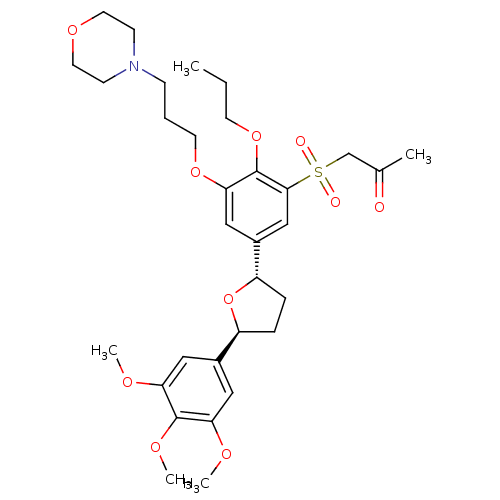
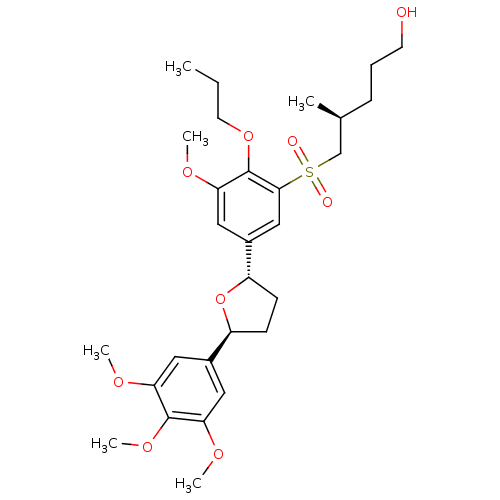
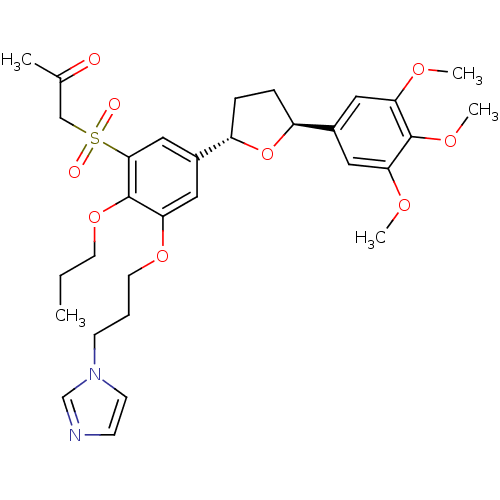
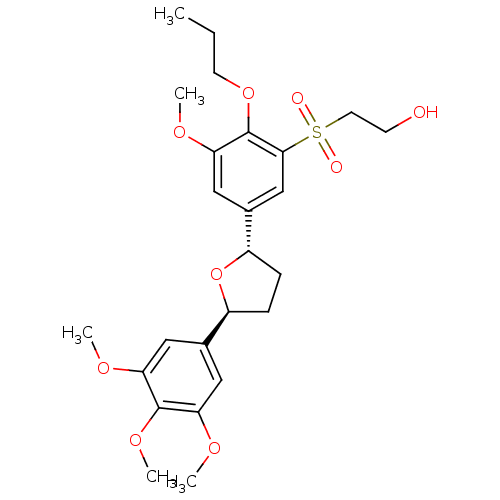
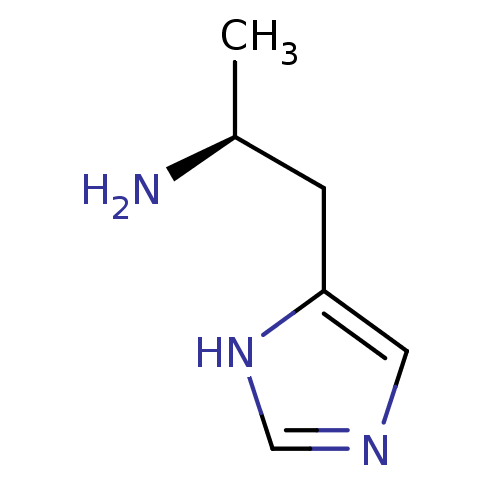
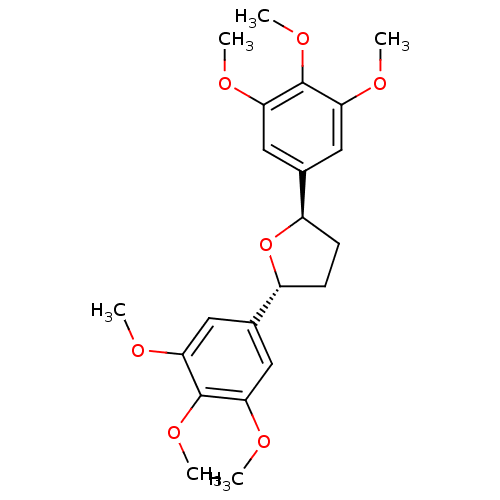
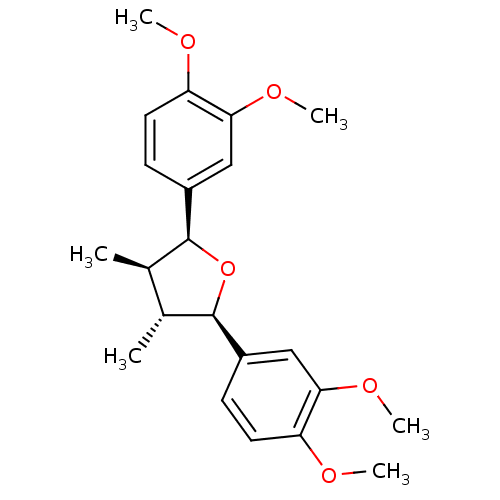
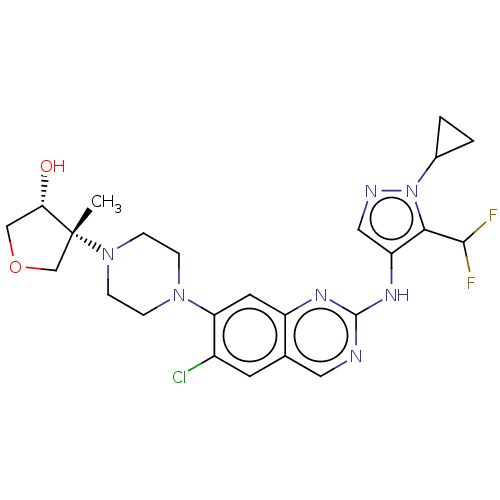
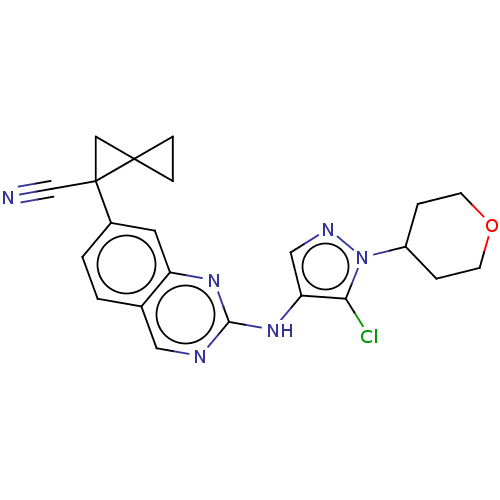
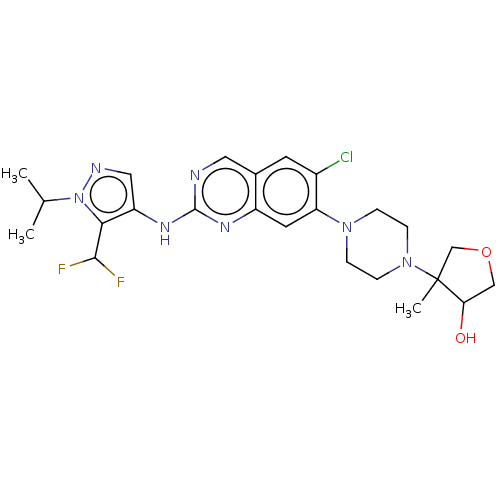
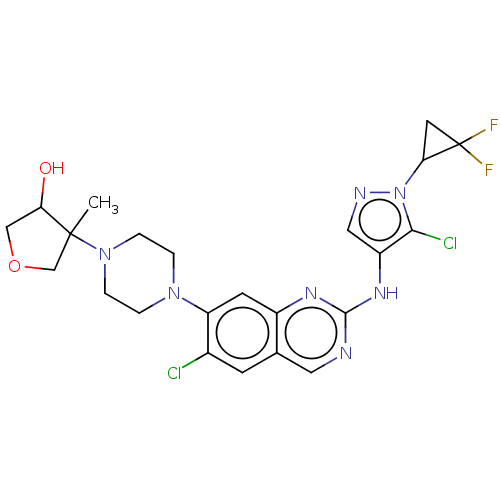
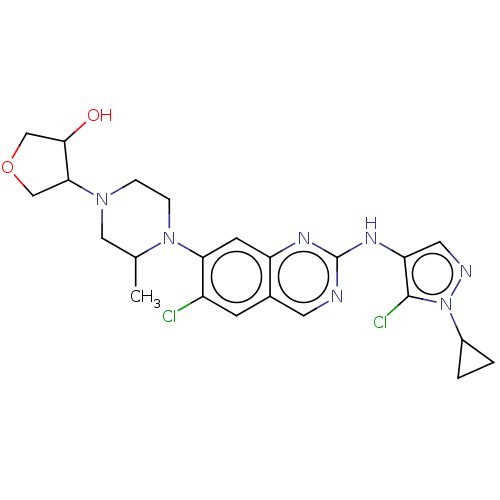
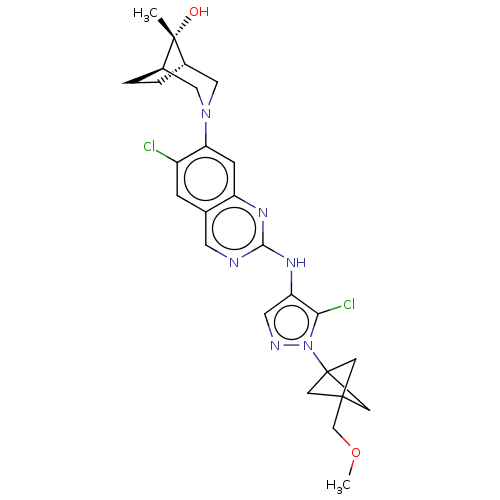
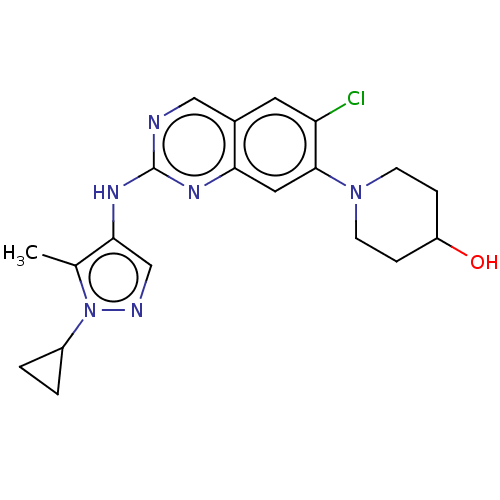
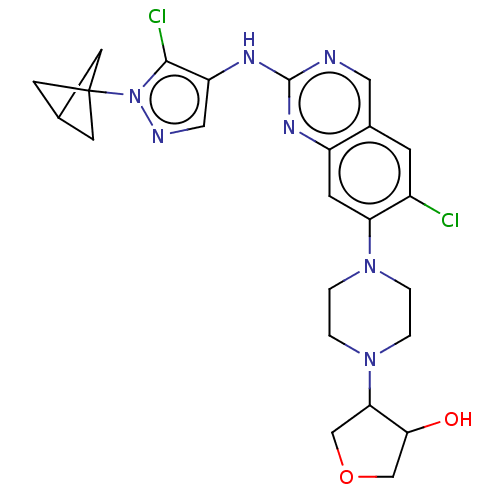
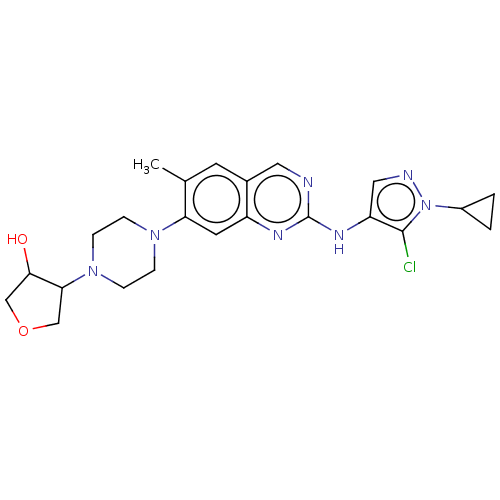
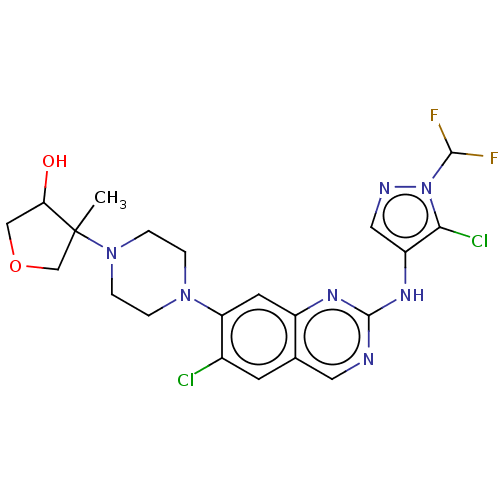
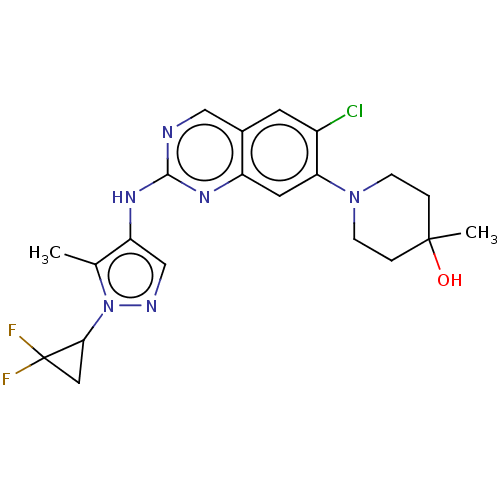
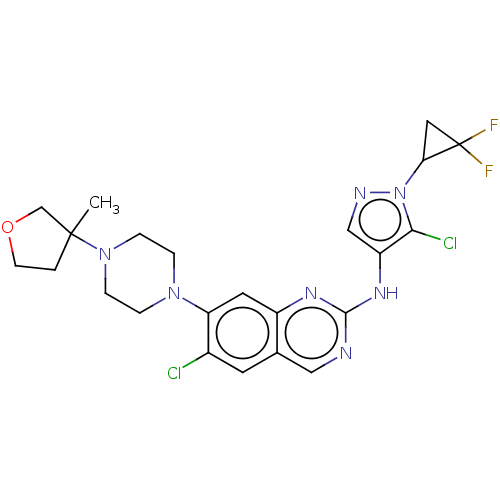
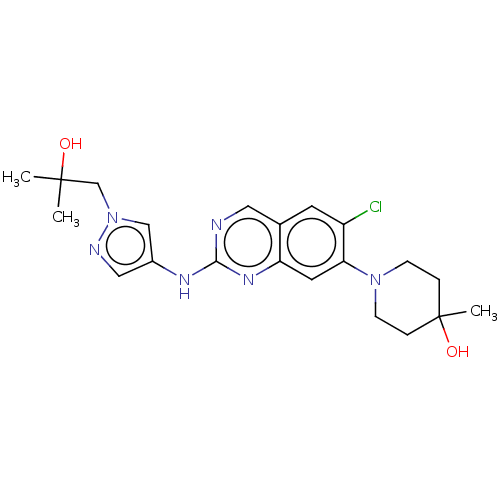
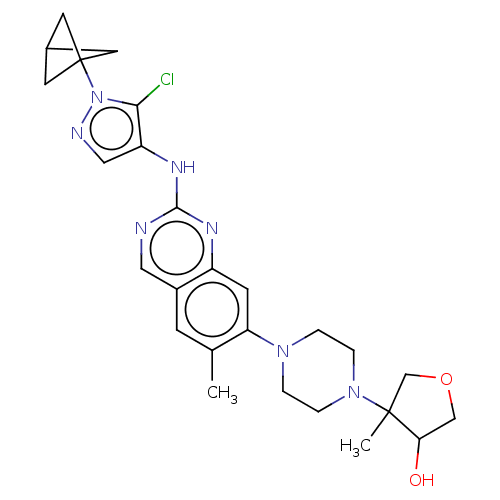
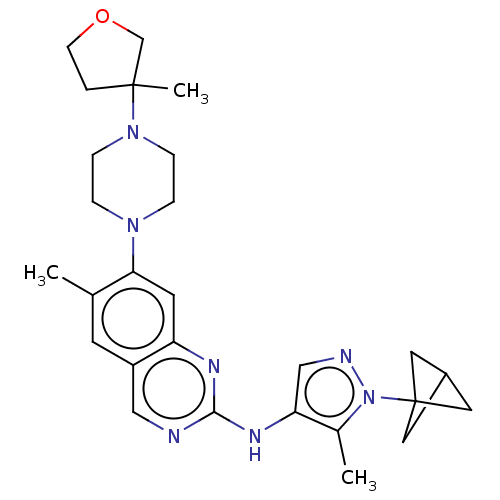
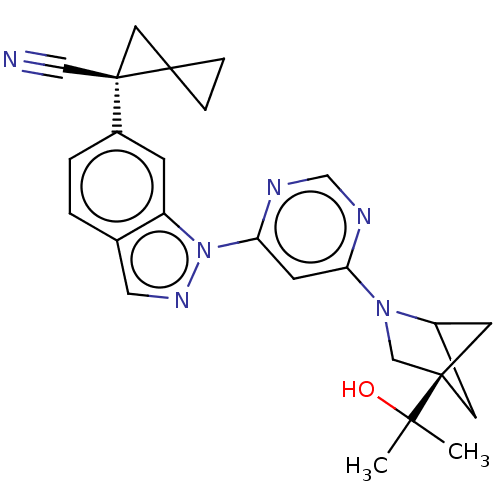
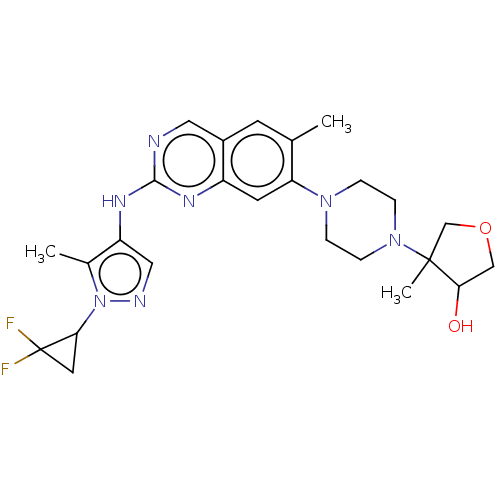
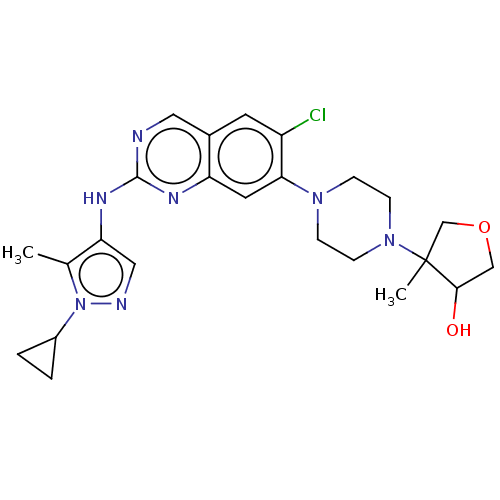
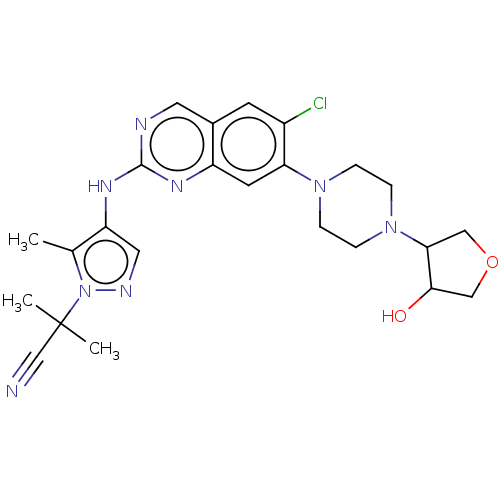
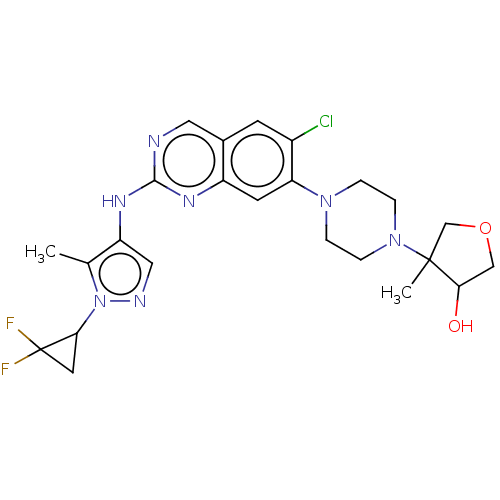
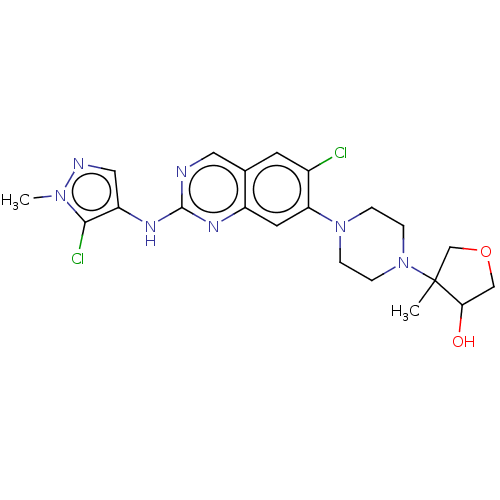
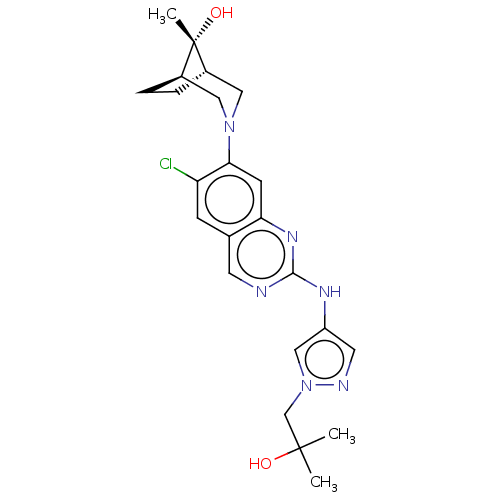
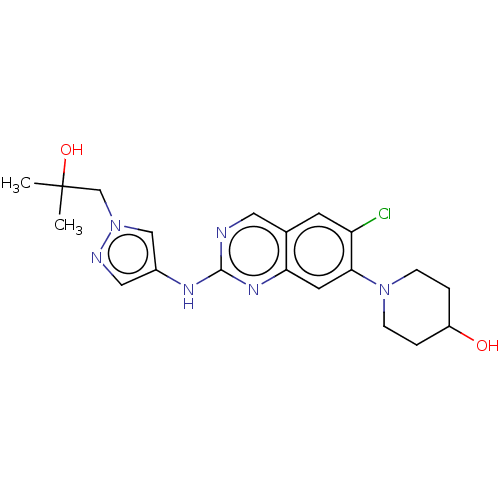
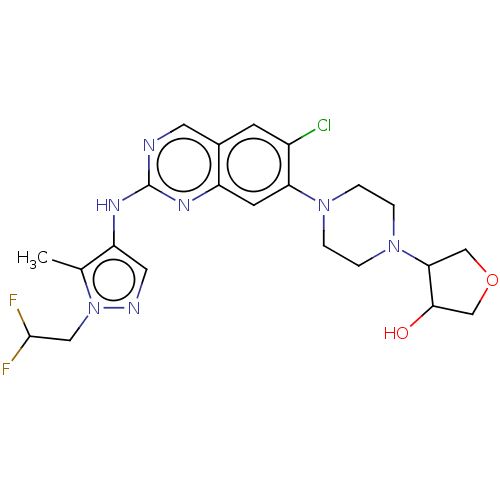
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.90nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.72nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 6.30nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 174nMAssay Description:In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of recombinant N-terminal GST-fused LRRK2 G2109S mutant (970 to 2527 residues) (unknown origin) preincubated with enzyme for 15 mins follo...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails