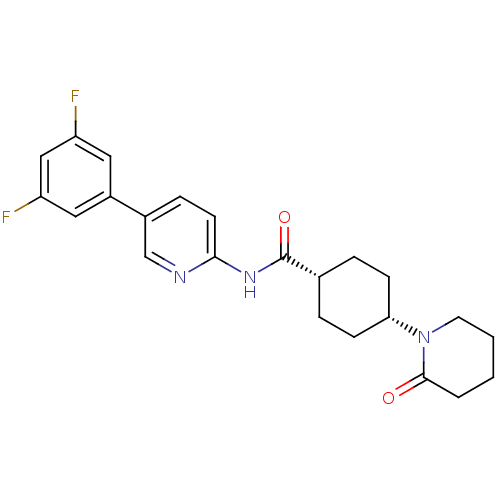
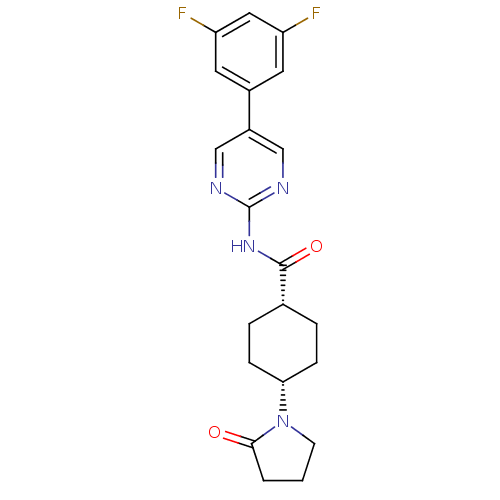
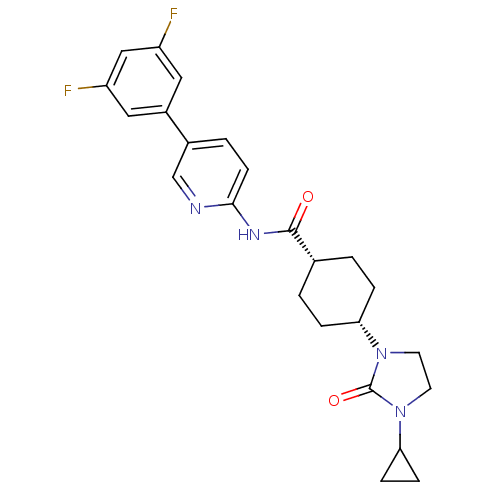
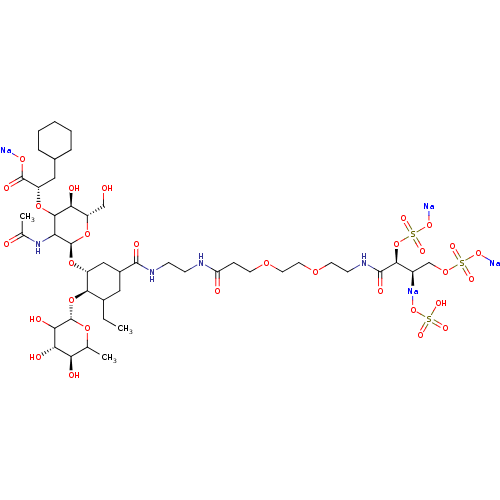
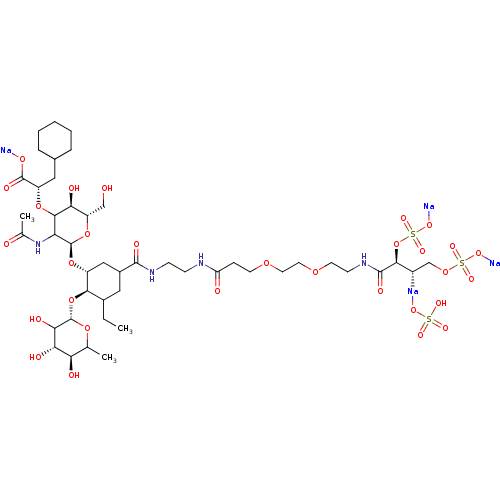
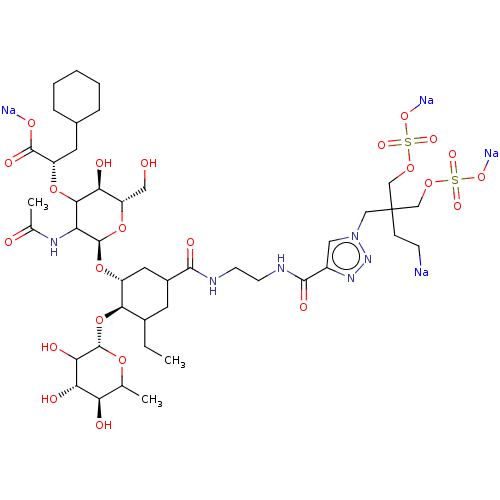
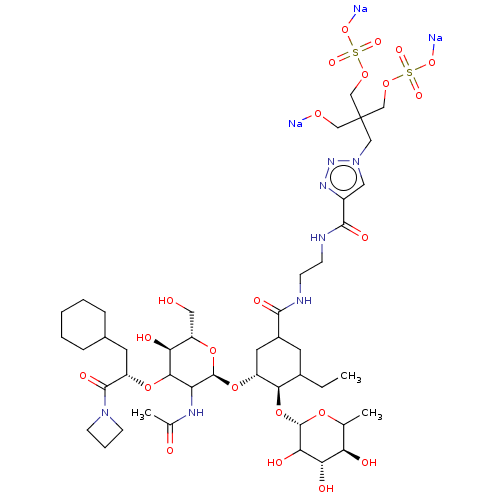
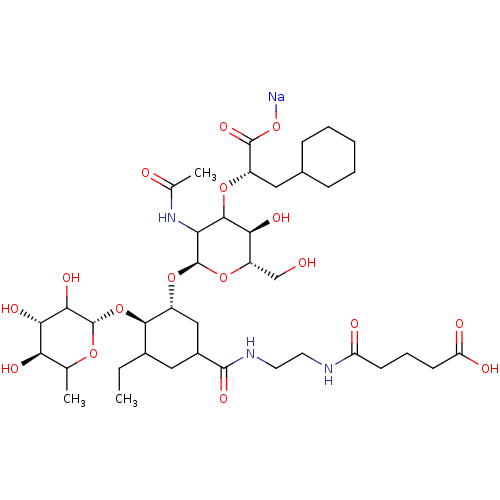
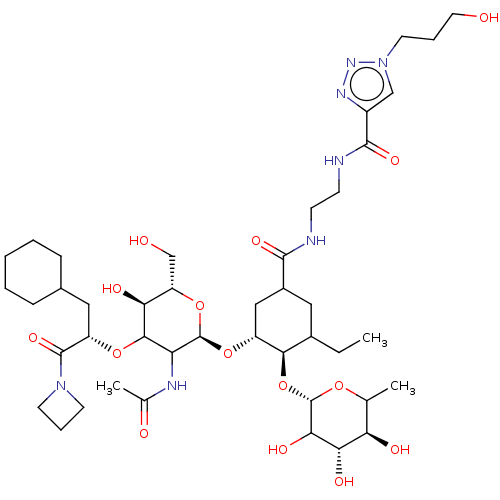
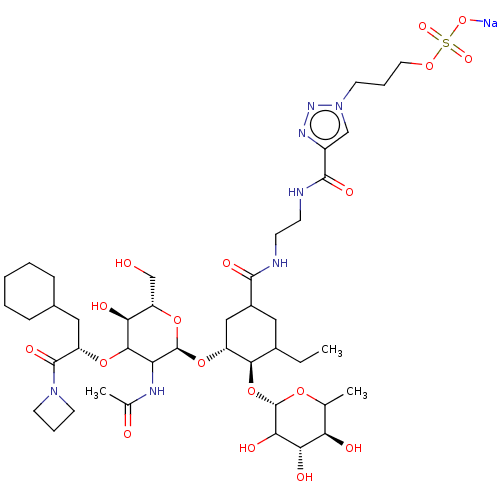
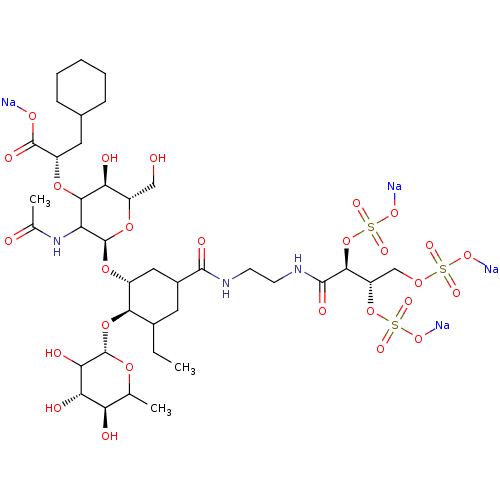
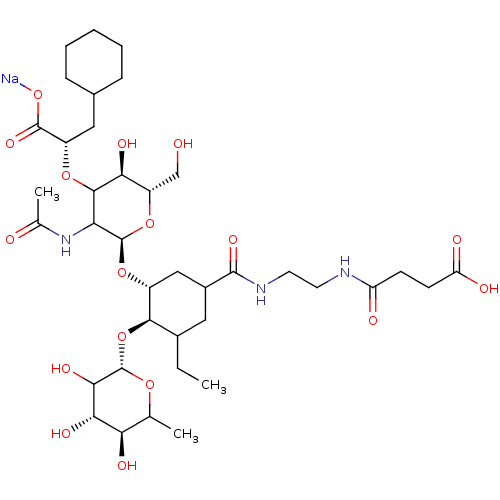
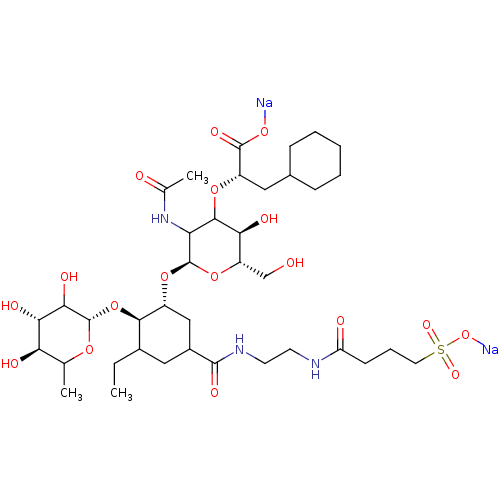
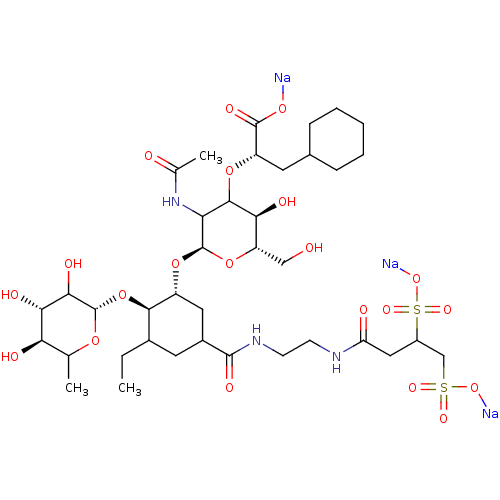
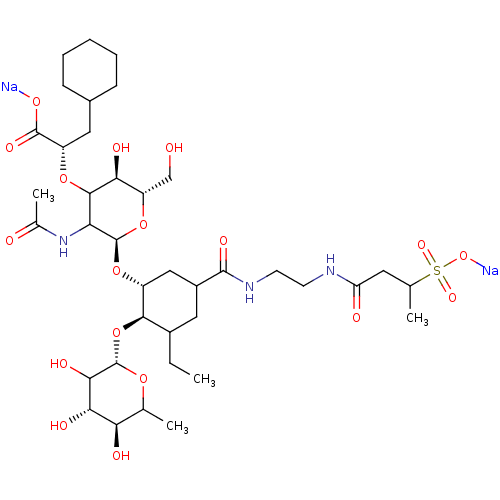
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
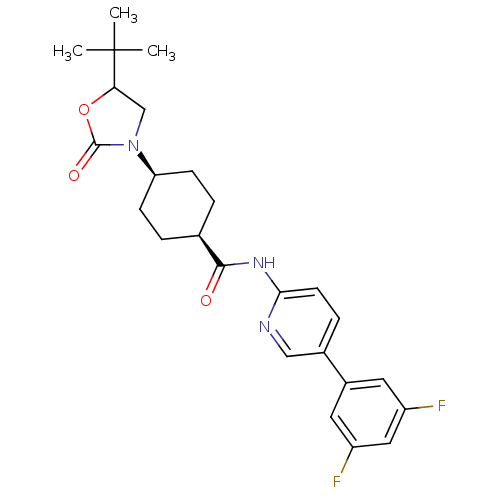
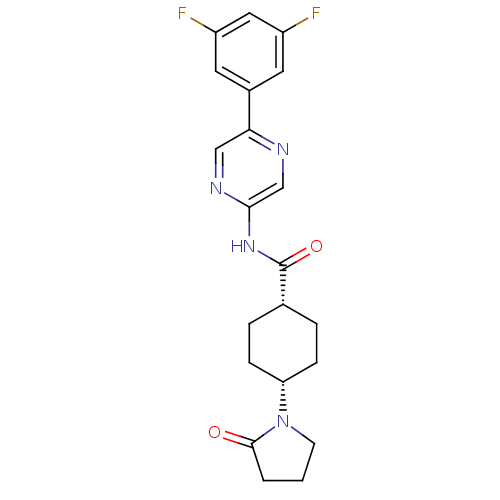
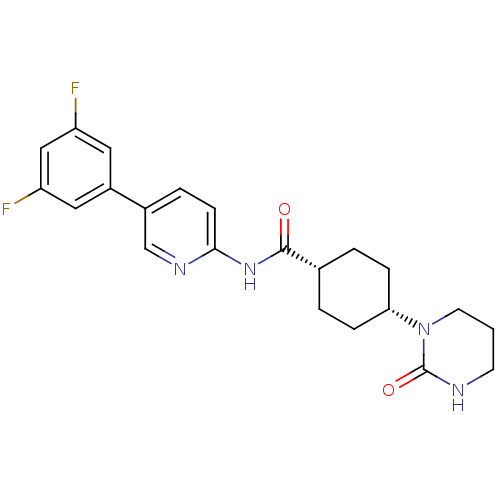
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
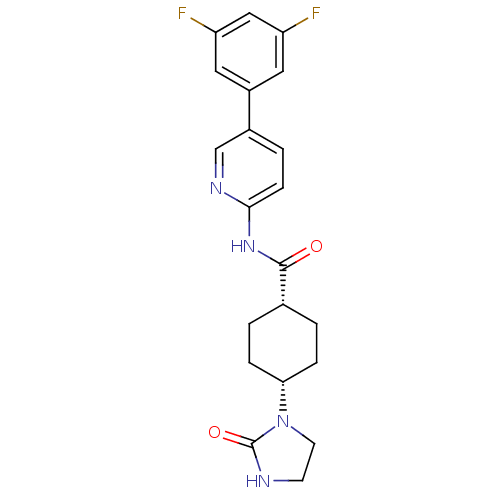
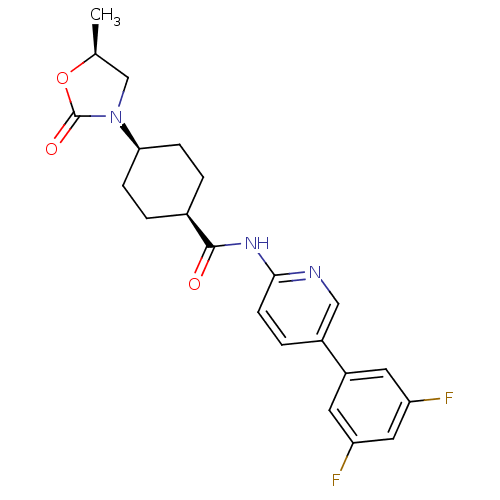
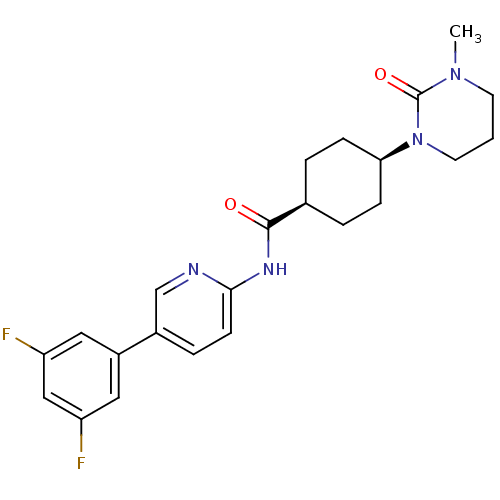
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
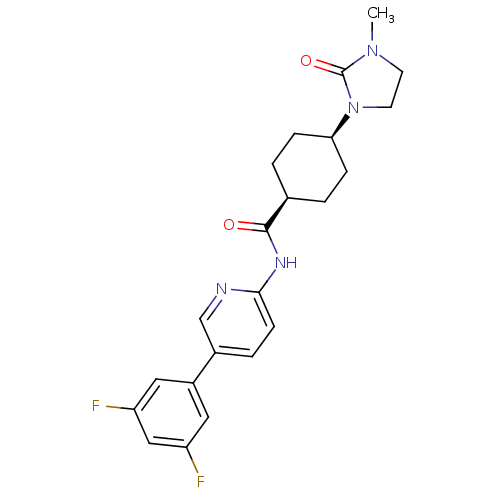
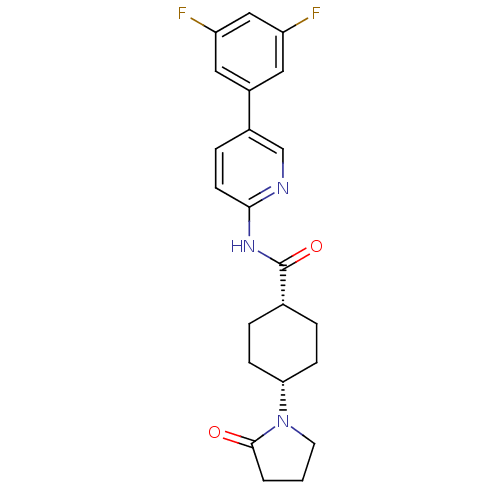
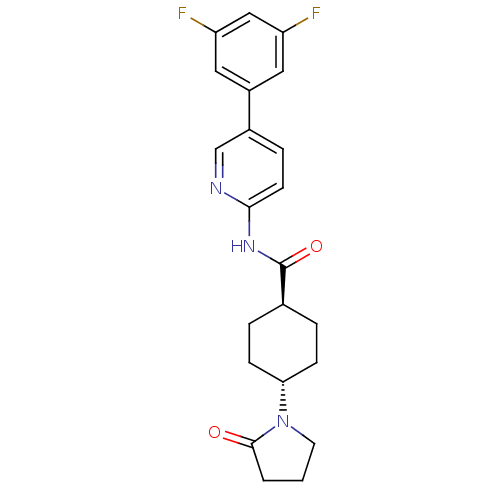
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 7nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: 720nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 5(Homo sapiens (Human))
Lundbeck Research USA, Inc.
Curated by ChEMBL
Lundbeck Research USA, Inc.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]PYY from human NPY5 receptor transfected in LM(tk-) cell membranes after 120 mins by solid scintillation countingMore data for this Ligand-Target Pair
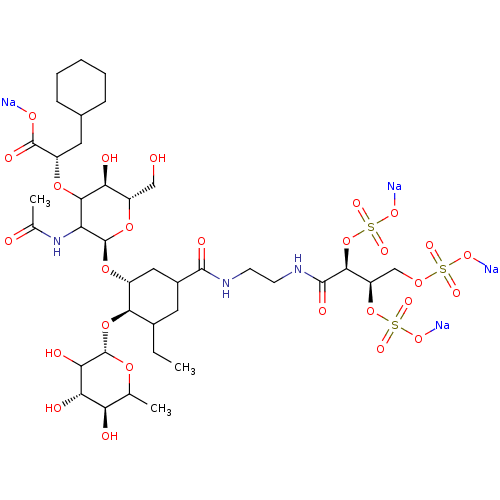
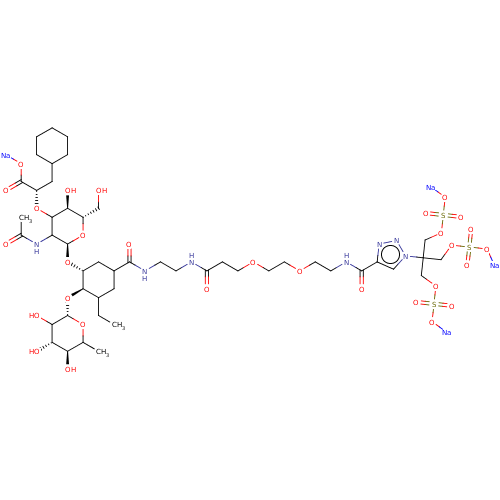
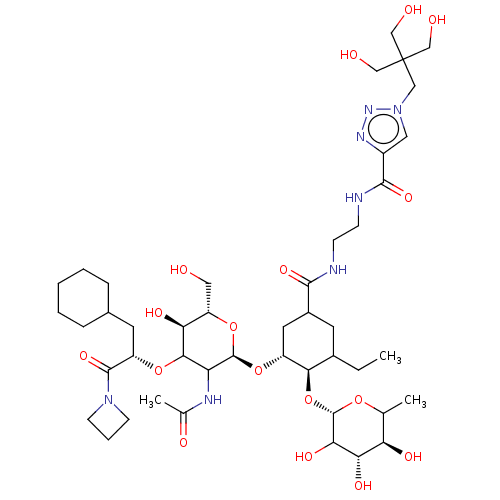
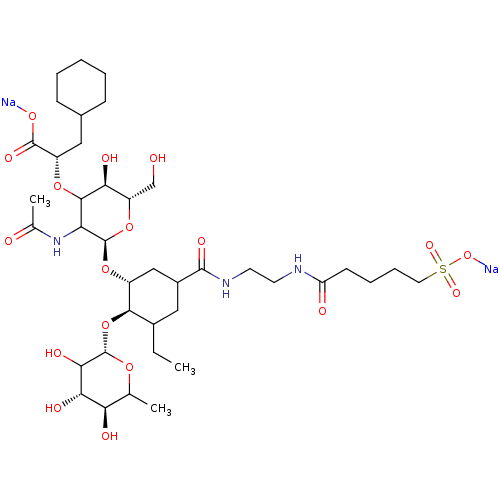
Affinity DataIC50: 106nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 136nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 192nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 520nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 660nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+3nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 2.36E+3nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair
Affinity DataIC50: 2.78E+3nMAssay Description:The inhibition assay to screen for and characterize glycomimetic antagonists of E-selectin is a competitive binding assay, which allows the determina...More data for this Ligand-Target Pair