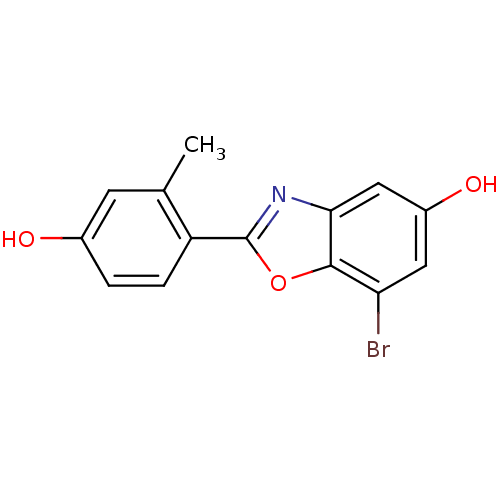
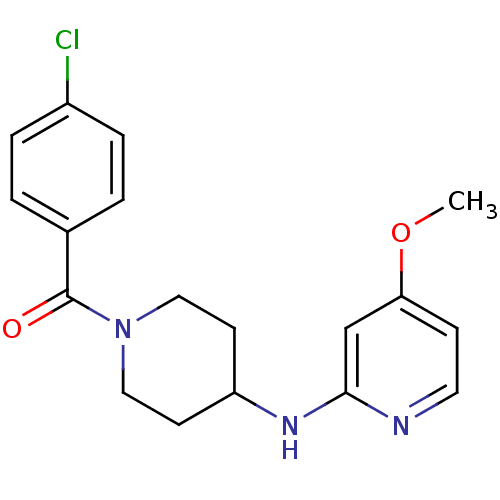
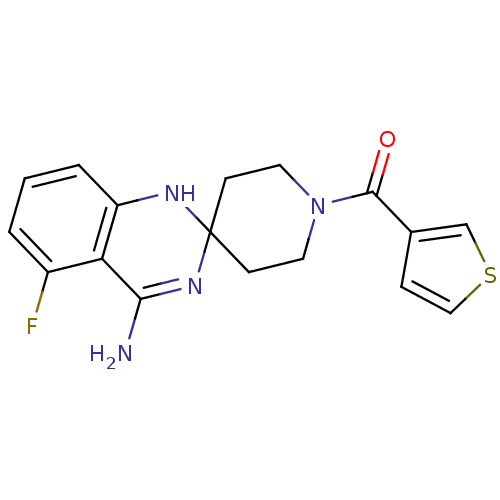
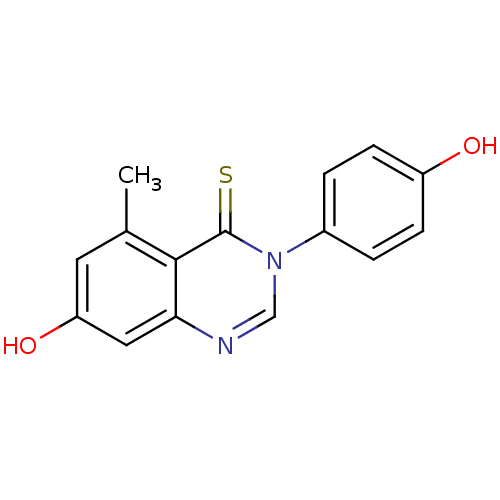
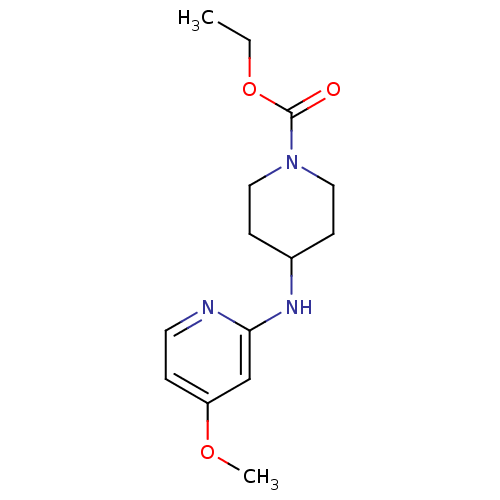
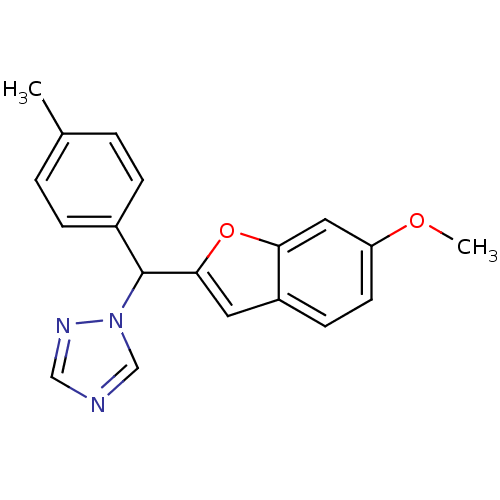
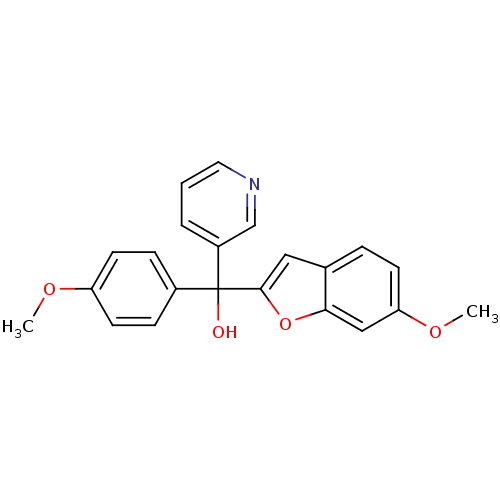
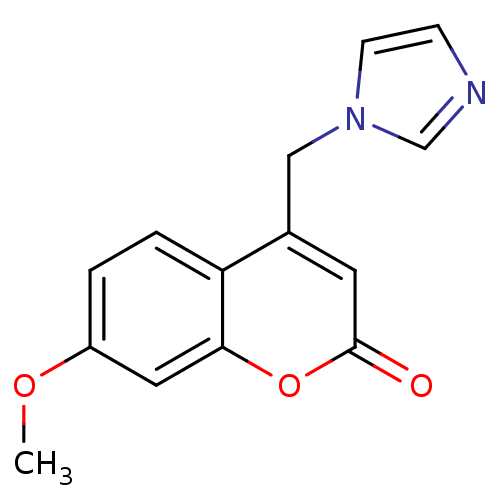
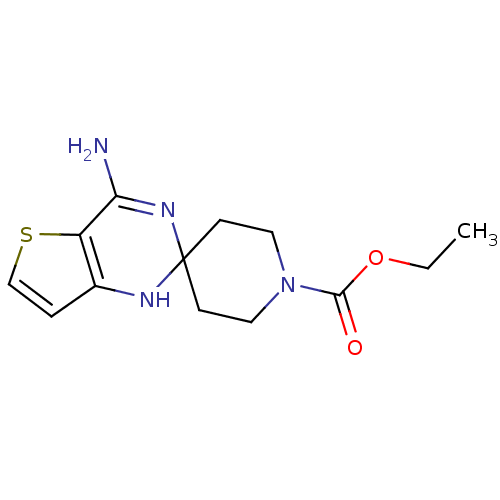
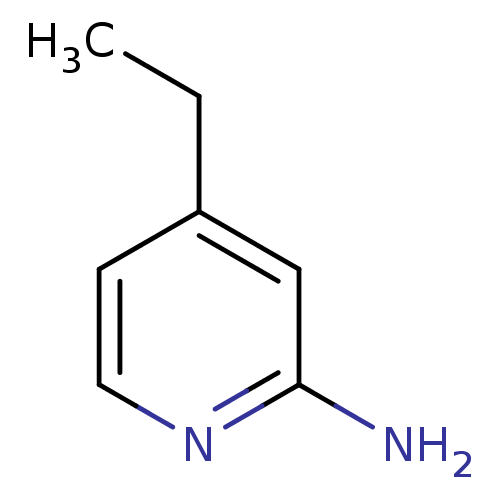
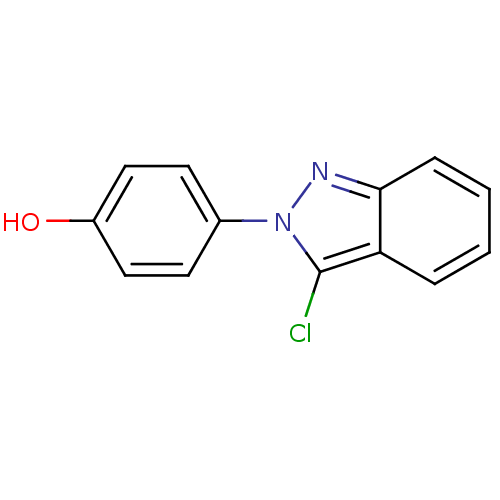
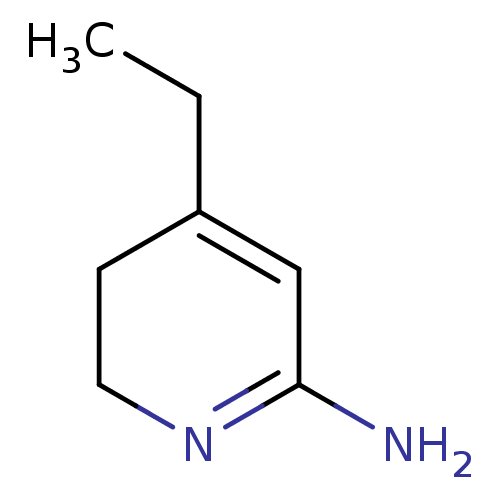
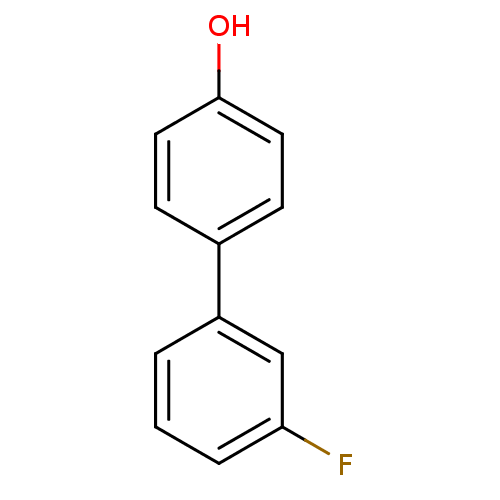
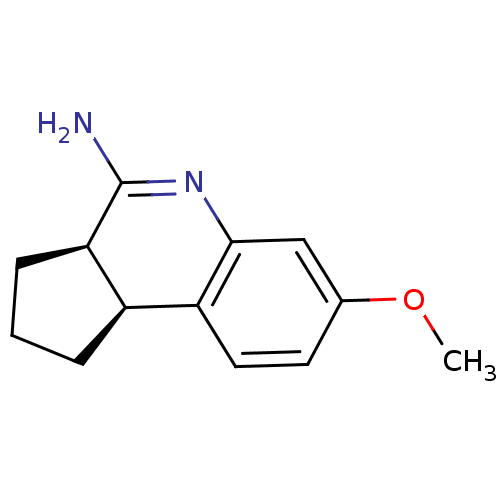
Affinity DataKi: 0.0230nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 0.790nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 1.47nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 2.79nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 4.67nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 5.46nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 7.30nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 10.7nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
Affinity DataKi: 14.5nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 34.1nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 64.5nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 95nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 119nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 176nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 237nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 253nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 299nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 645nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 654nMAssay Description:Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranesMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 772nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataKi: 1.43E+3nMAssay Description:Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cellsMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of estrogen receptor betaMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of estrogen receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscleMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscleMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of estrogen receptor betaMore data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Antagonism of the [Ca2+] efflux actions of the human Bradykinin receptor B2 (SK-N-SH neuroblastoma)More data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (WI38 fibroblasts)More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Antagonism of the [Ca2+] efflux actions of the human Bradykinin receptor B2 (WI38 fibroblasts)More data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 69nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 79nMAssay Description:Inhibition of estrogen receptor betaMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
TargetB2 bradykinin receptor(Homo sapiens (Human))
Novartis Institute for Medical Sciences
Curated by ChEMBL
Novartis Institute for Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 91nMAssay Description:Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (SK-N-SH neuroblastoma)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
Affinity DataIC50: 105nMAssay Description:Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2More data for this Ligand-Target Pair
Affinity DataIC50: 141nMAssay Description:Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Inhibition of aromataseMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of iNOSMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of estrogen receptor betaMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of iNOSMore data for this Ligand-Target Pair
TargetEstrogen receptor beta(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of estrogen receptor betaMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of iNOSMore data for this Ligand-Target Pair

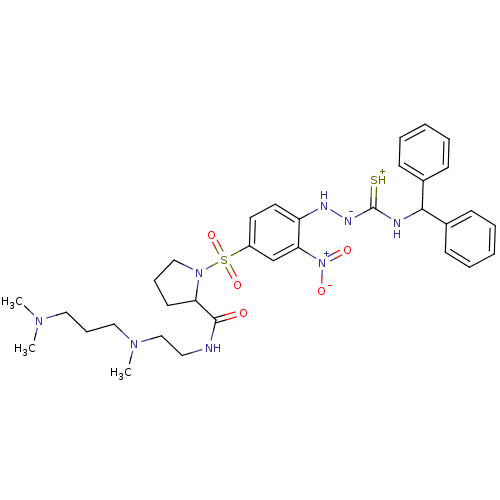
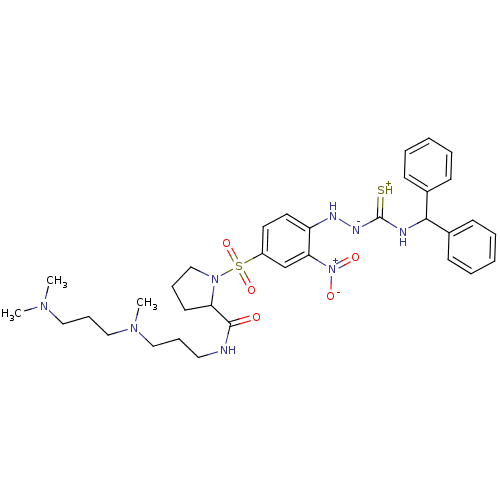
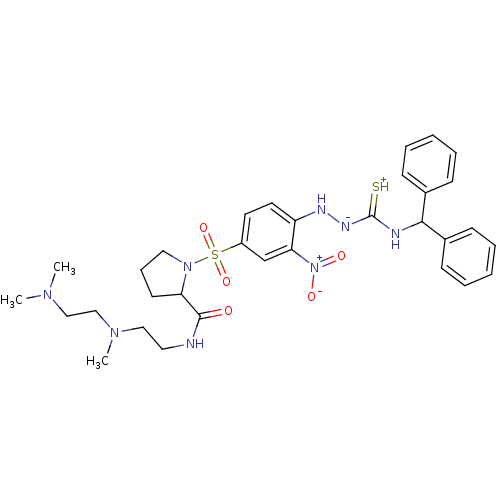
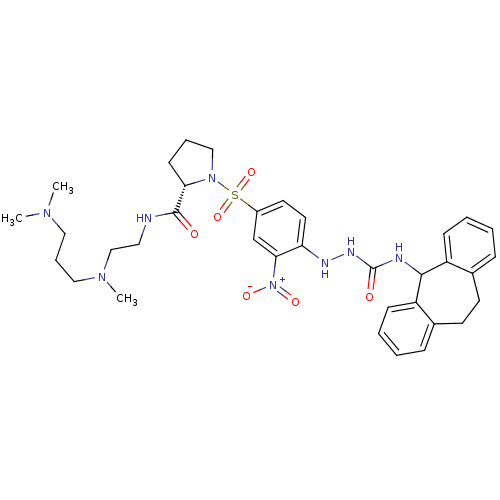
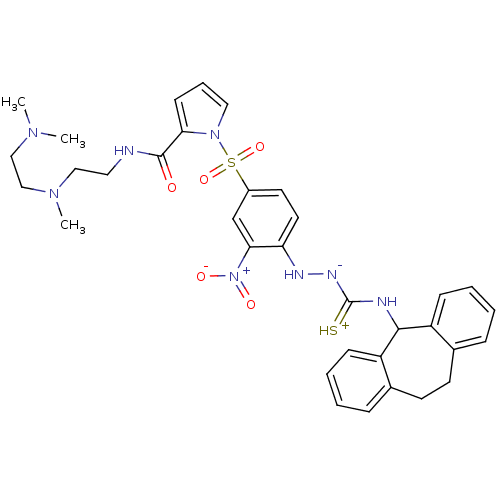
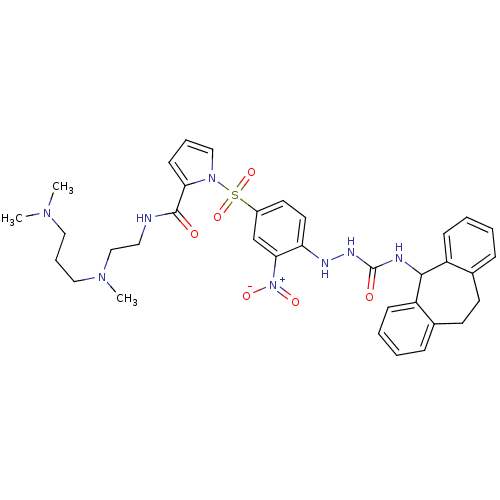
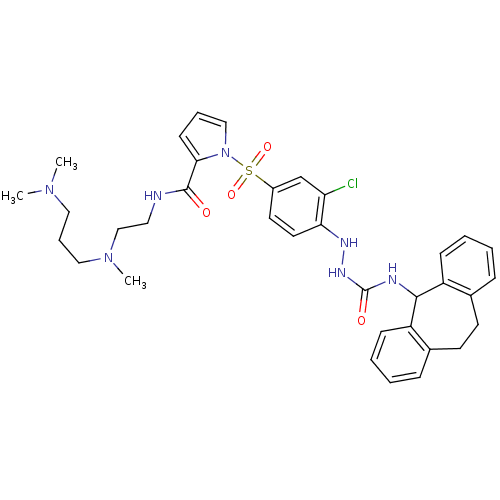
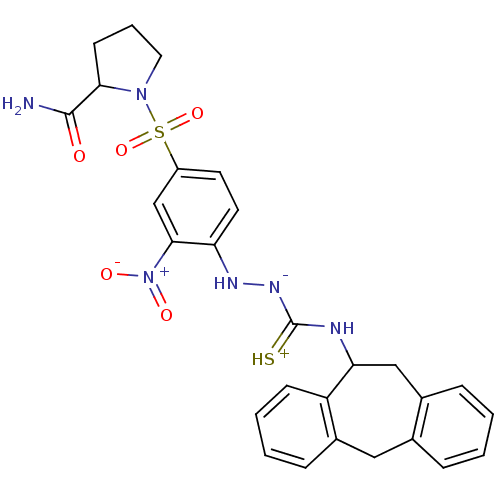
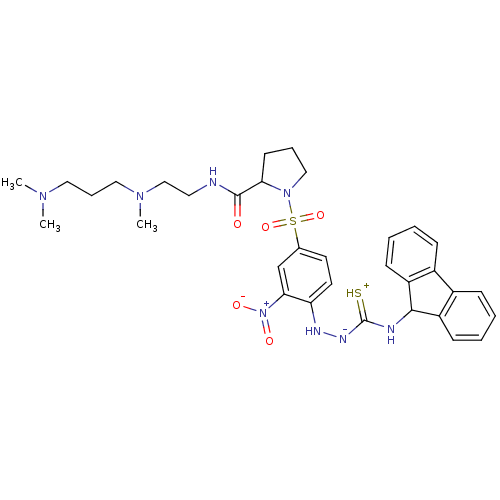
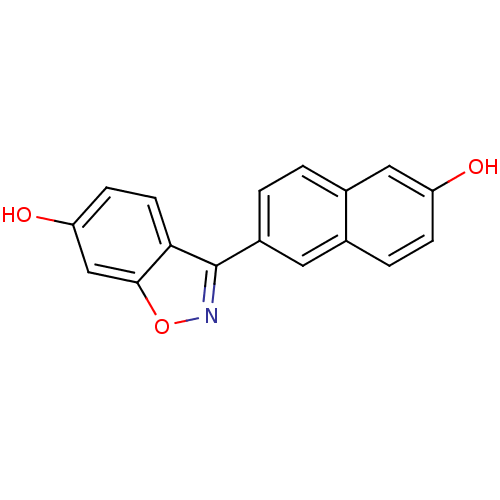
 3D Structure (crystal)
3D Structure (crystal)