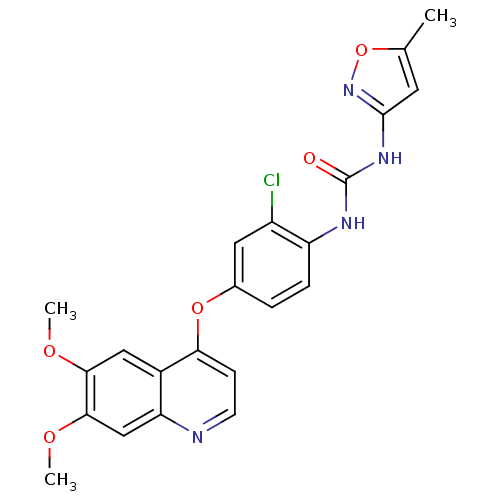
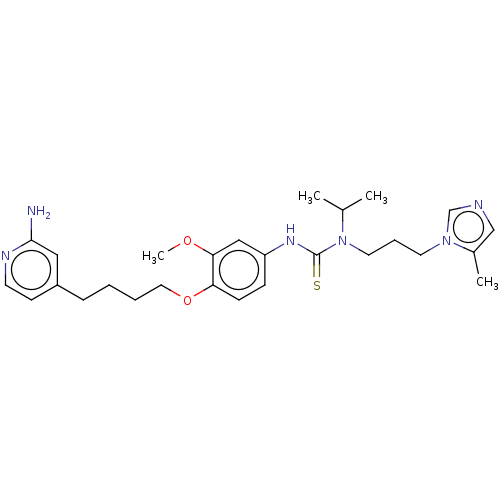
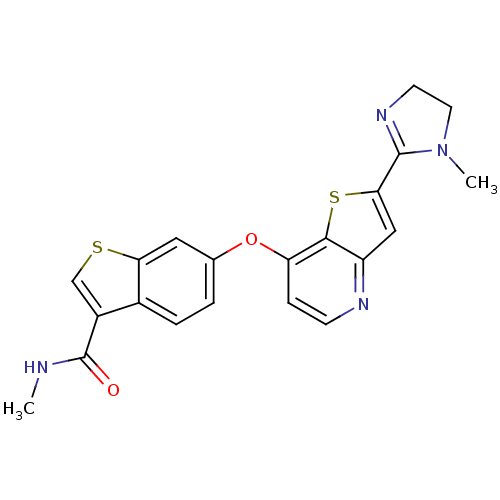
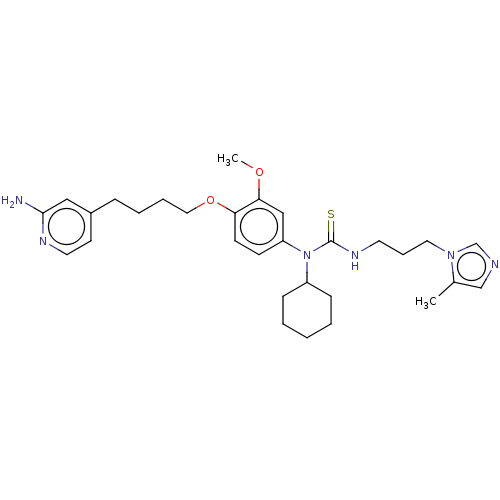
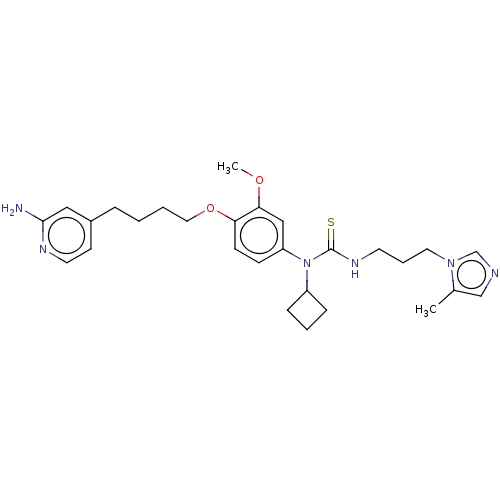
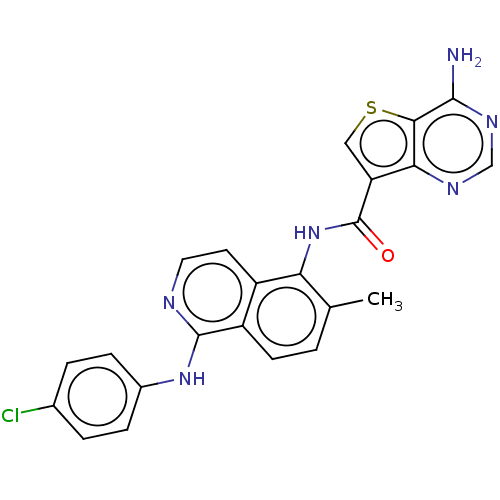
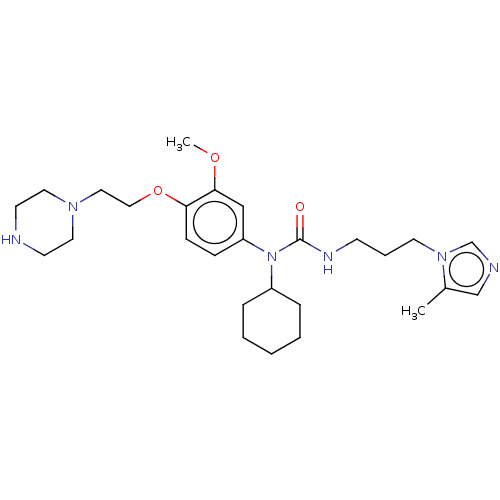
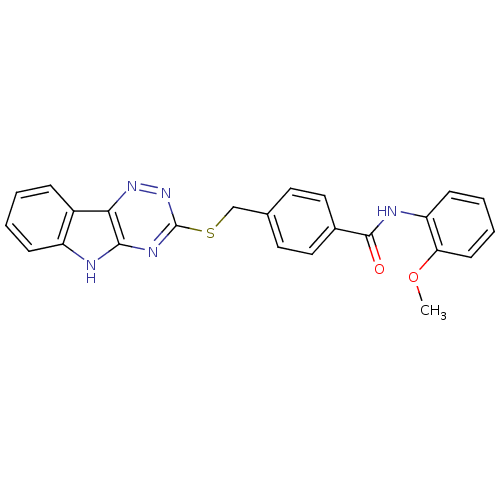
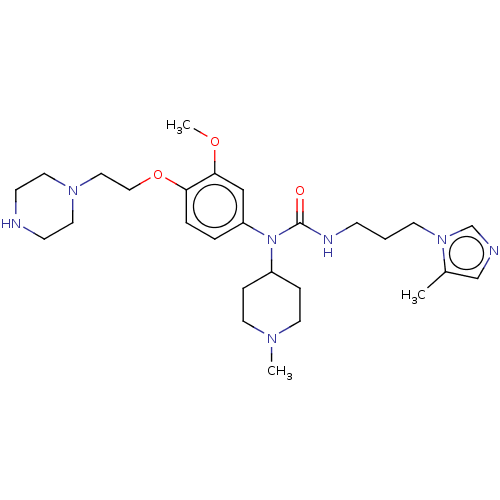
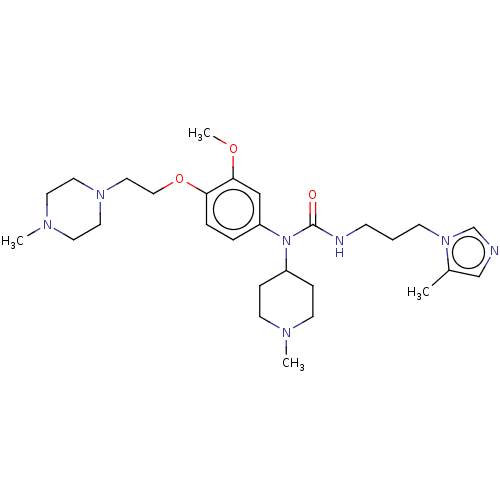
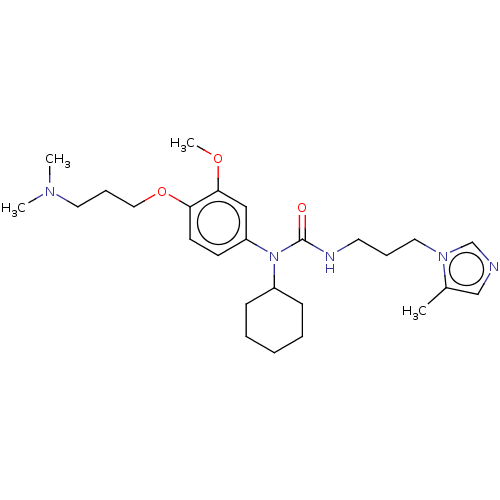
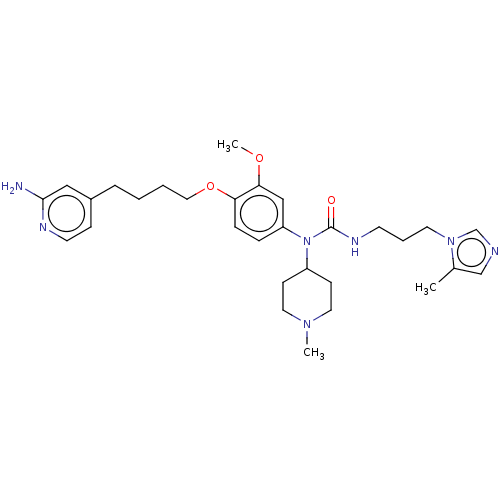
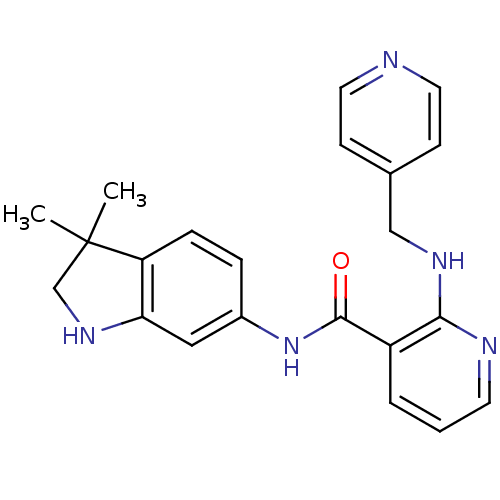
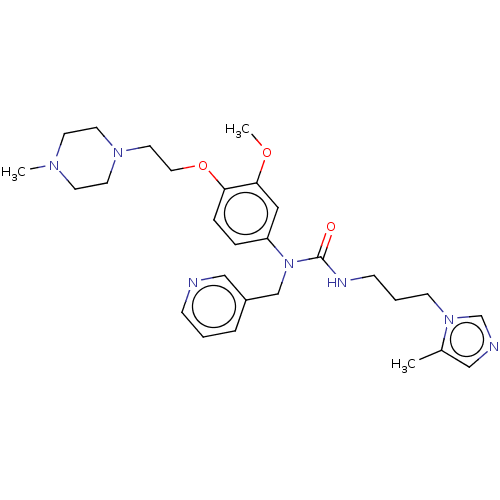
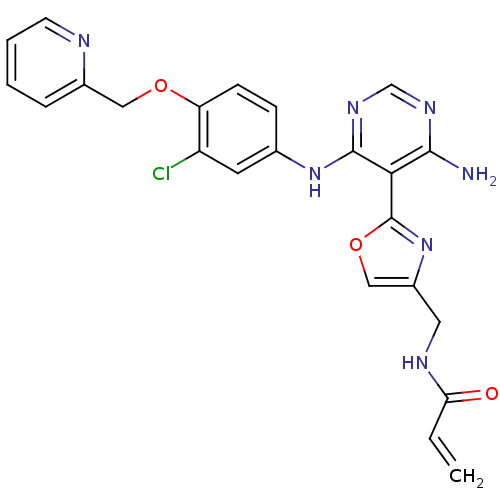
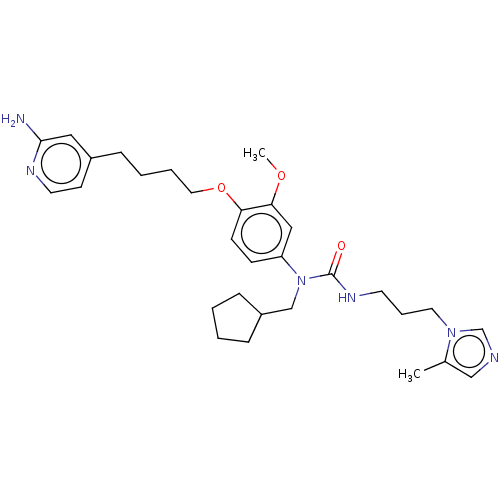
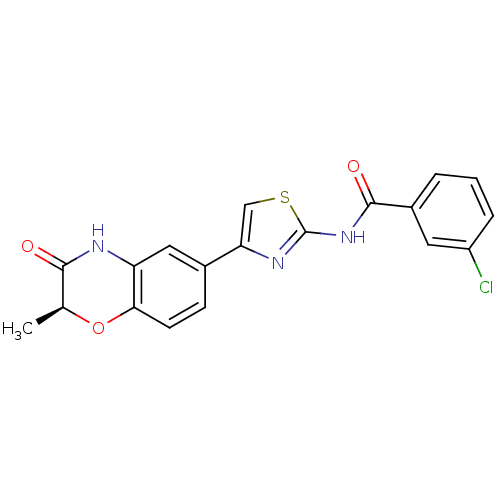
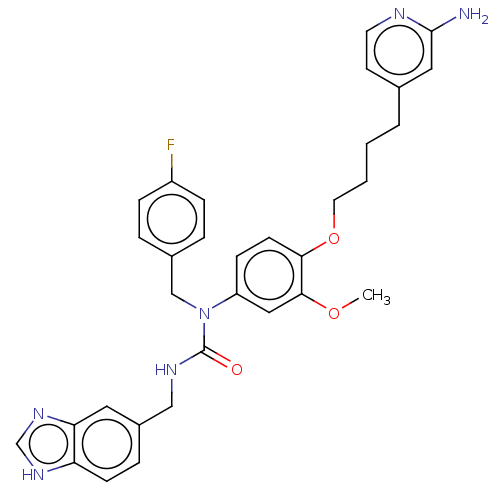
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
TargetfMet-Leu-Phe receptor(Homo sapiens (Human))
Pohang University of Science and Technology
Curated by PDSP Ki Database
Pohang University of Science and Technology
Curated by PDSP Ki Database
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 0.160nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
HANMI PHARM. CO., LTD
US Patent
HANMI PHARM. CO., LTD
US Patent
Affinity DataIC50: 1nMAssay Description:The compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profiling Servic...More data for this Ligand-Target Pair
TargetMacrophage colony-stimulating factor 1 receptor(Homo sapiens (Human))
HANMI PHARM. CO., LTD
US Patent
HANMI PHARM. CO., LTD
US Patent
Affinity DataIC50: 1nMAssay Description:As such, the compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profili...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Src kinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetEpithelial discoidin domain-containing receptor 1(Homo sapiens (Human))
SRMLSC
Curated by PubChem BioAssay
SRMLSC
Curated by PubChem BioAssay
Affinity DataIC50: 2nMAssay Description:As such, the compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profili...More data for this Ligand-Target Pair
TargetEpithelial discoidin domain-containing receptor 1(Homo sapiens (Human))
SRMLSC
Curated by PubChem BioAssay
SRMLSC
Curated by PubChem BioAssay
Affinity DataIC50: 2nMAssay Description:The compounds prepared in Examples were tested for inhibitory activity against FMS, DDR1 and DDR2 kinases using Kinase Screening and Profiling Servic...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Src kinase (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of Src kinase (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Inhibition of wild-type EGFR preincubated for 10 mins followed by incubation for 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Konkuk University
Curated by ChEMBL
Konkuk University
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of recombinant VEGFR2 after 1 hr by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human recombinant glutaminyl cyclase using H-Gln-AMC hydrobromide as substrate measured after 15 mins by fluorimetryMore data for this Ligand-Target Pair

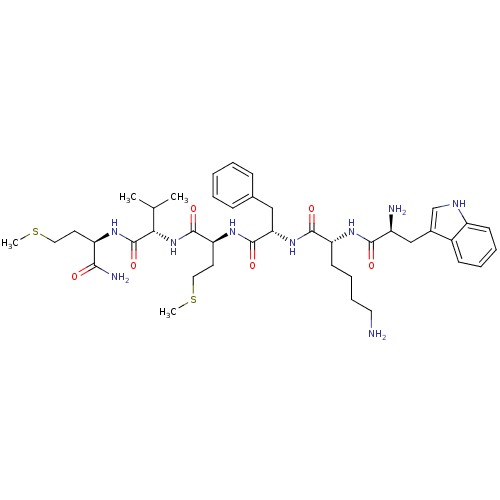
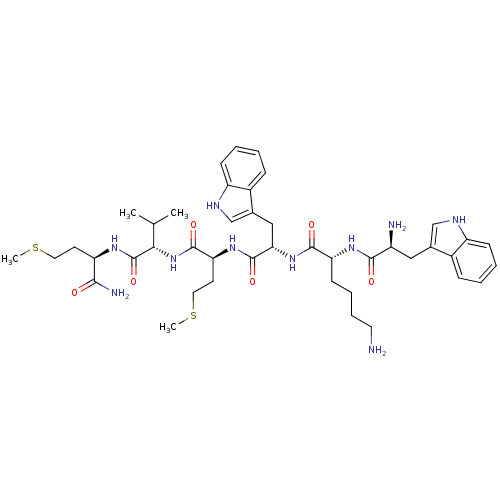
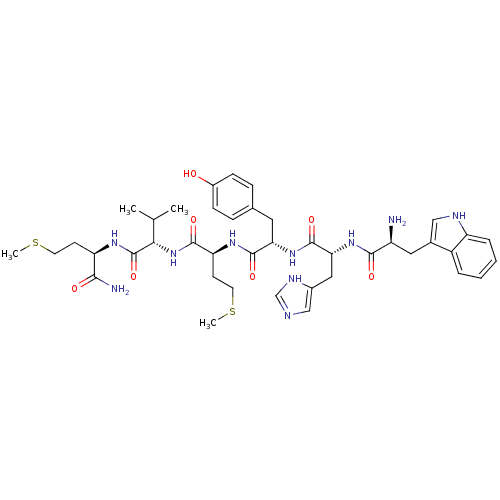
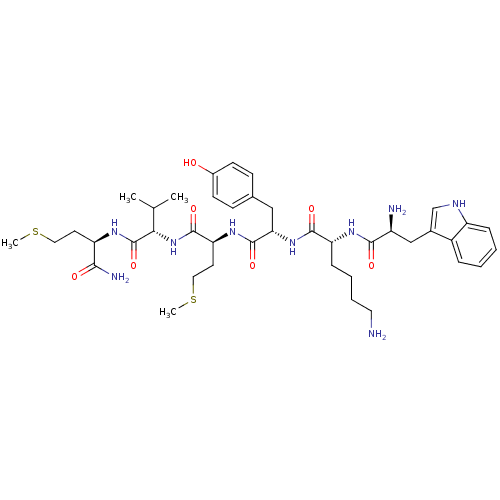
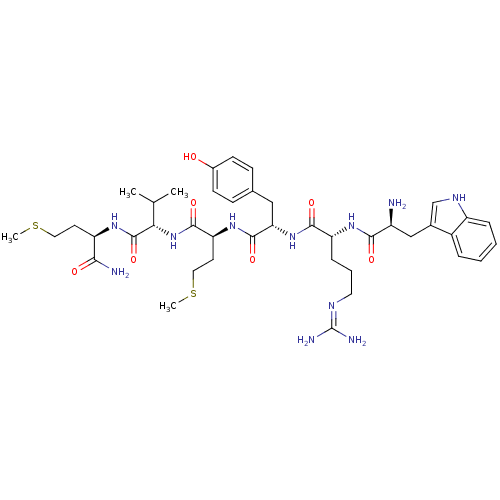
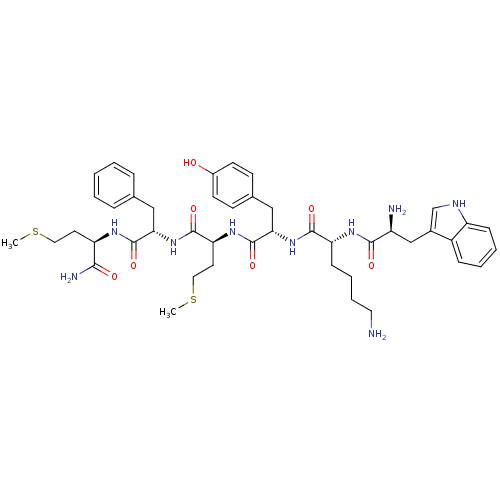
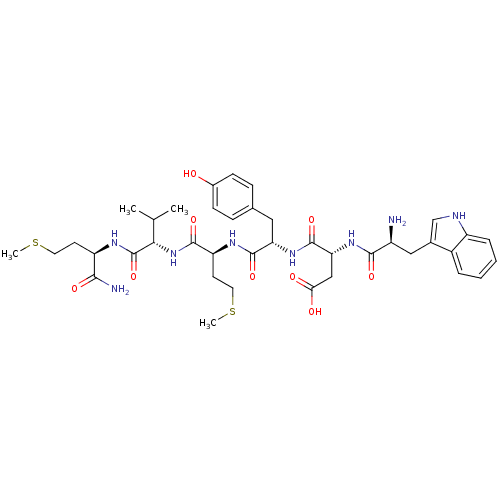
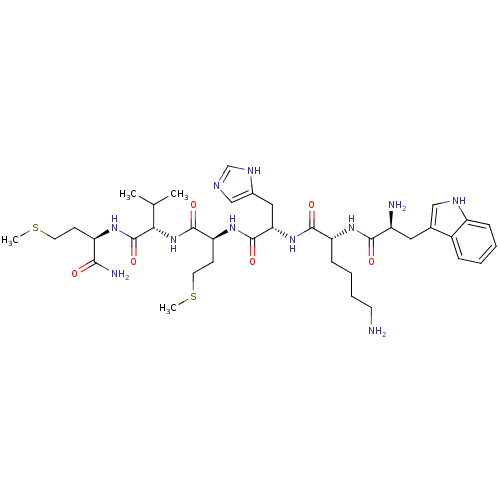
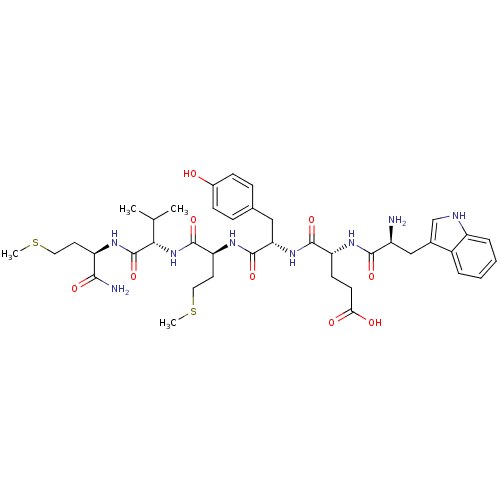
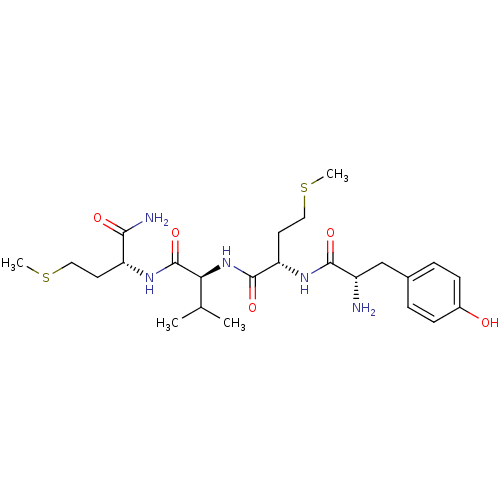
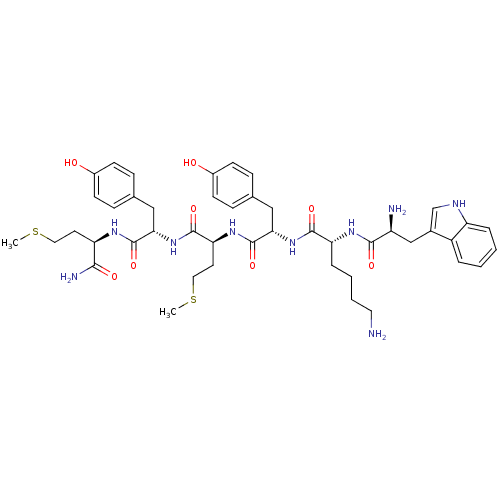
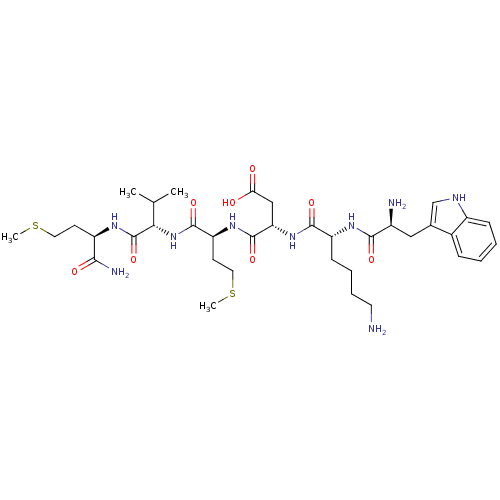
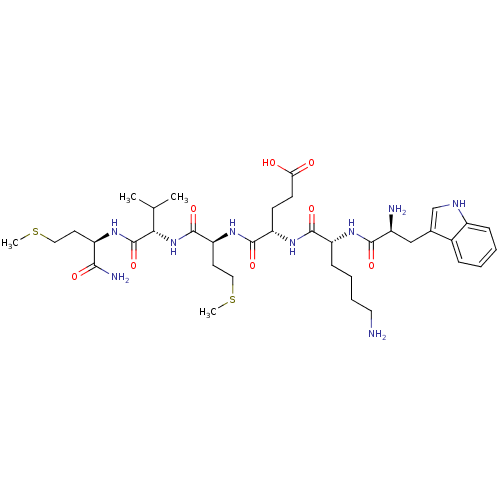

 3D Structure (crystal)
3D Structure (crystal)