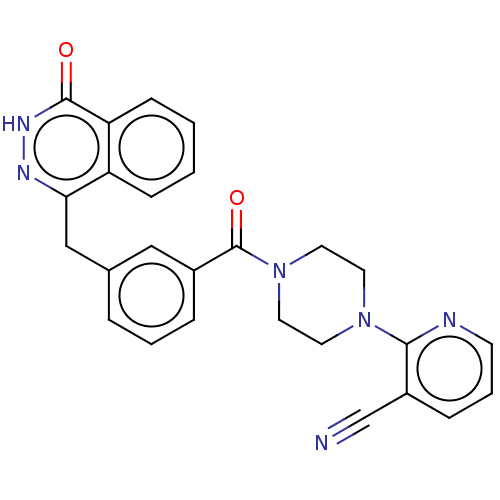
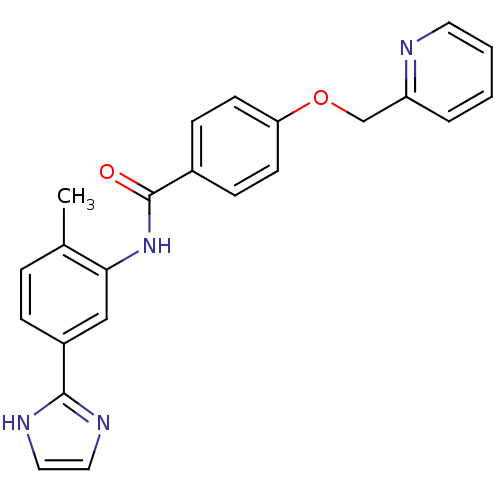
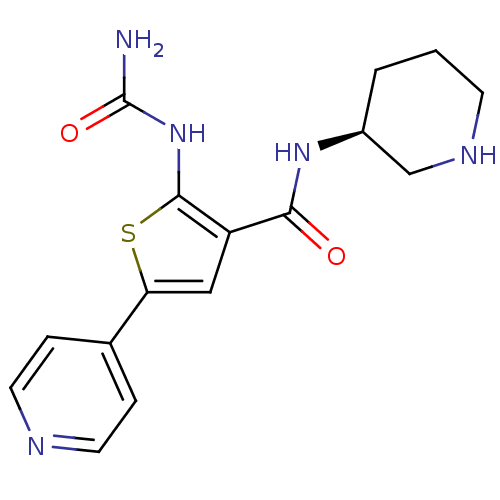
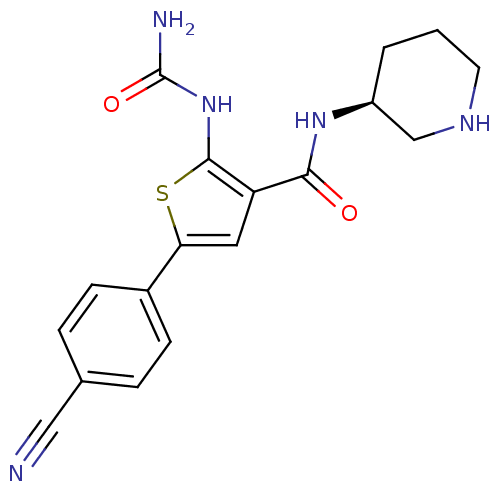
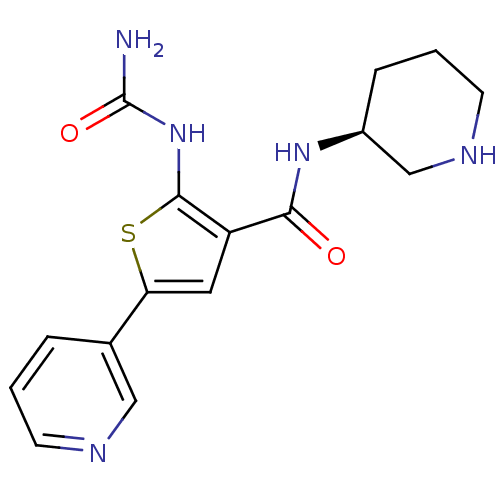
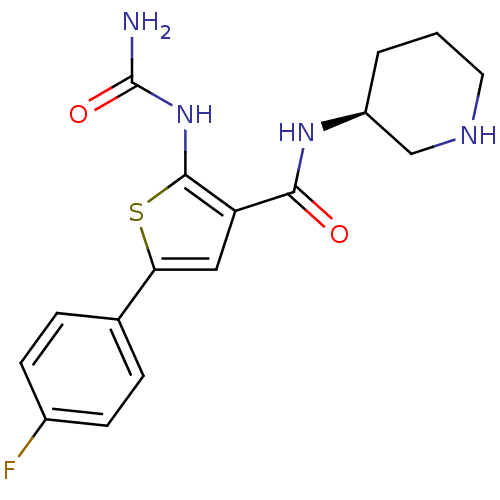
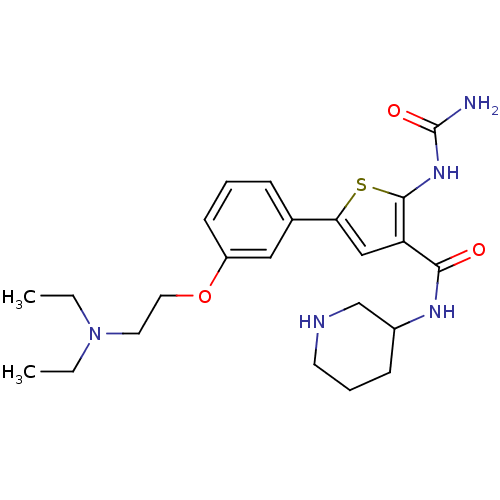
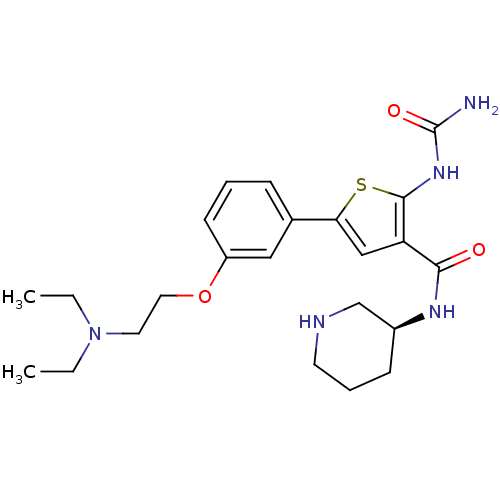
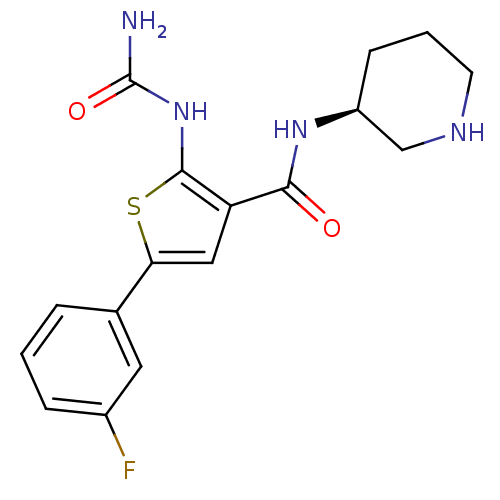
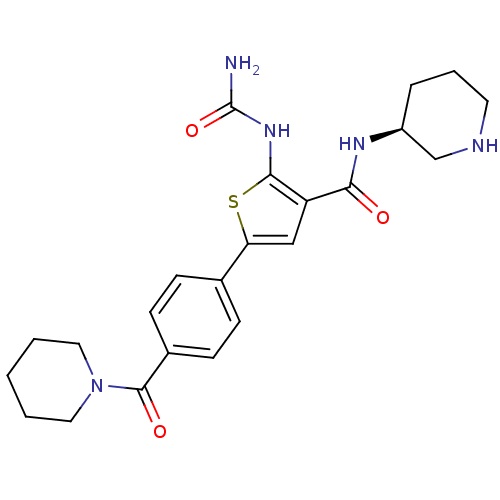
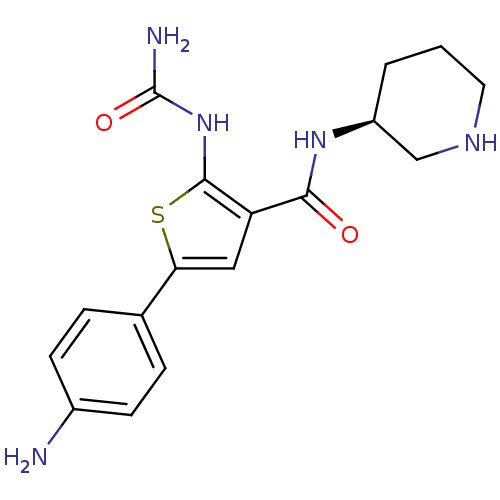
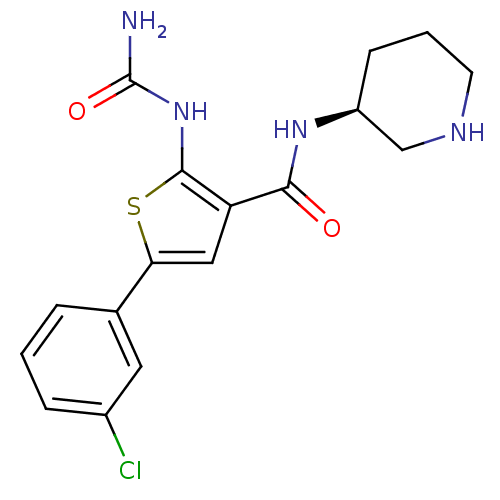
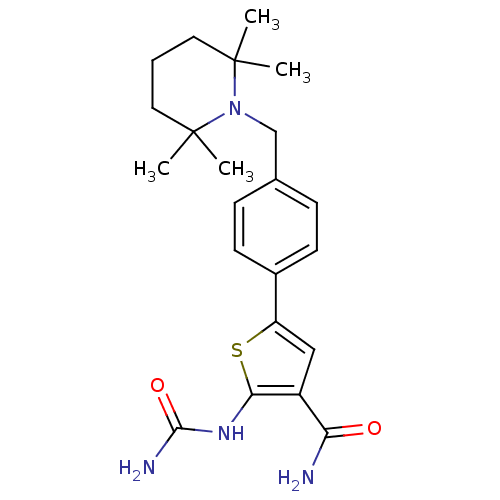
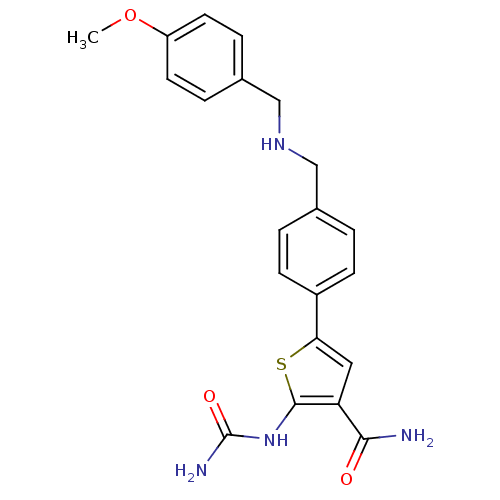
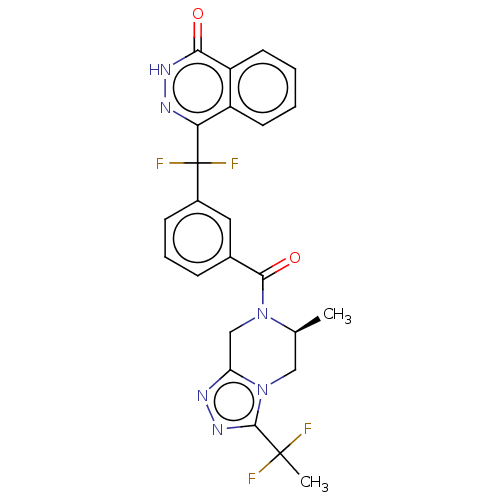
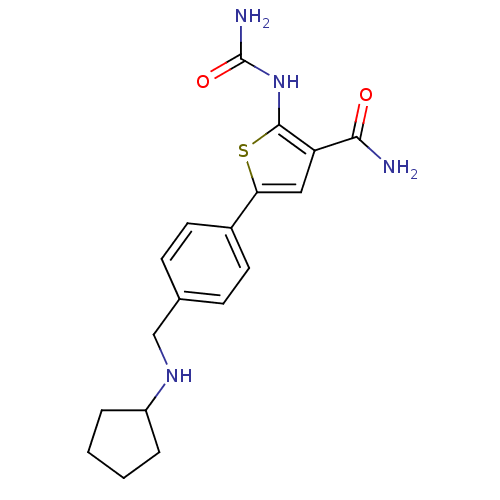
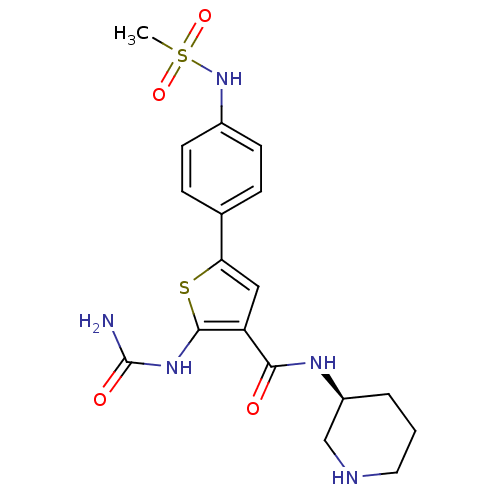
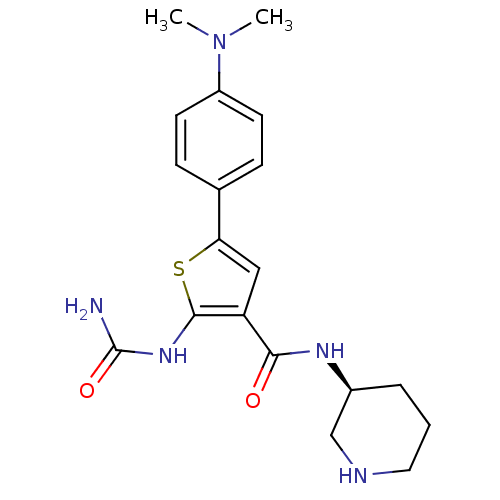
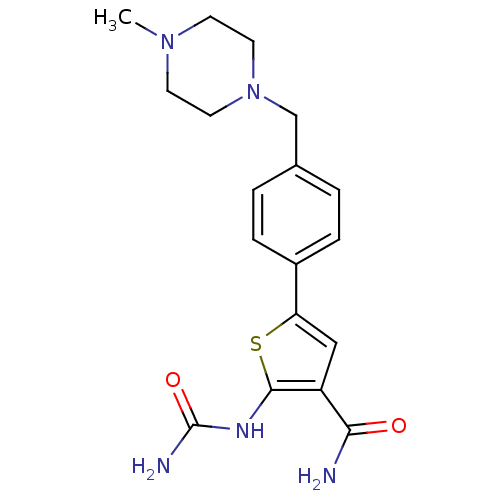
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
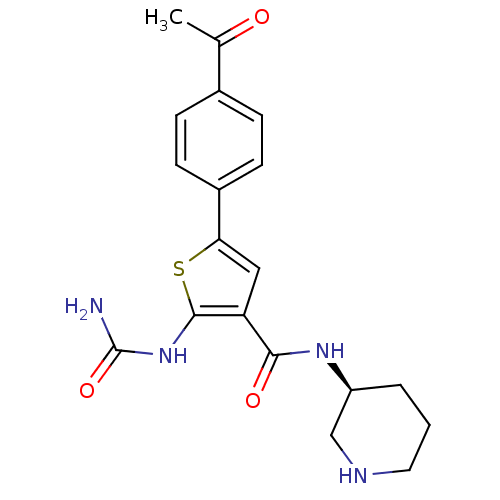
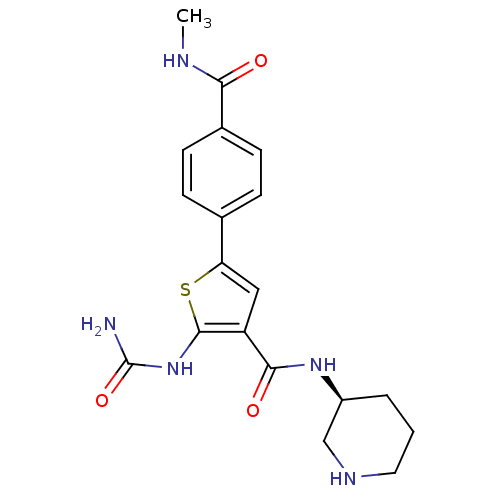
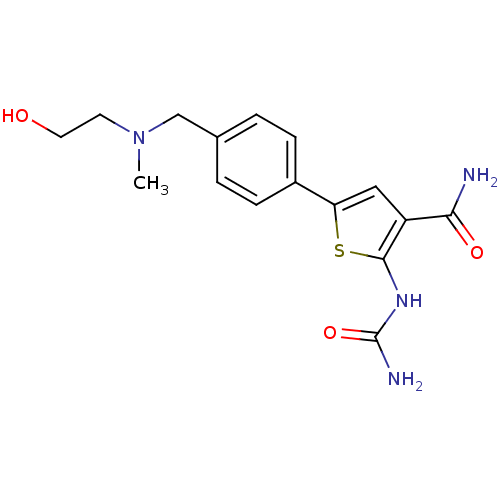
Affinity DataIC50: 0.200nMAssay Description:Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
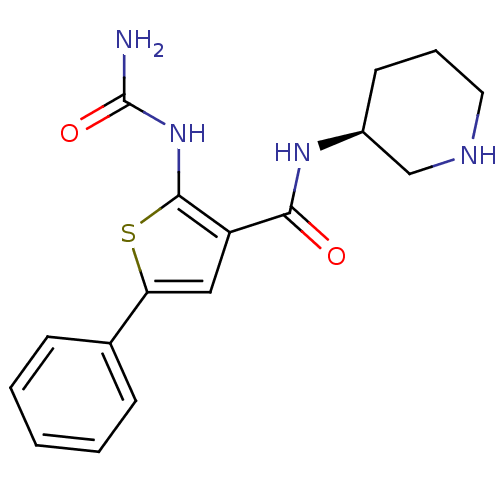
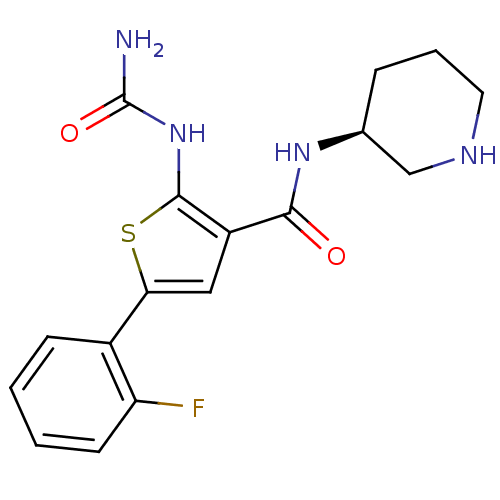
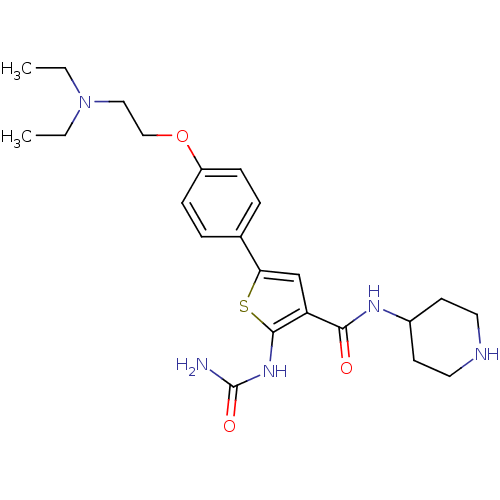
Affinity DataIC50: 0.400nMAssay Description:Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of full length human PARP1 expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidin-...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human PARP2 (2 to 583 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by strep...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of full length human PARP1 expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidin-...More data for this Ligand-Target Pair
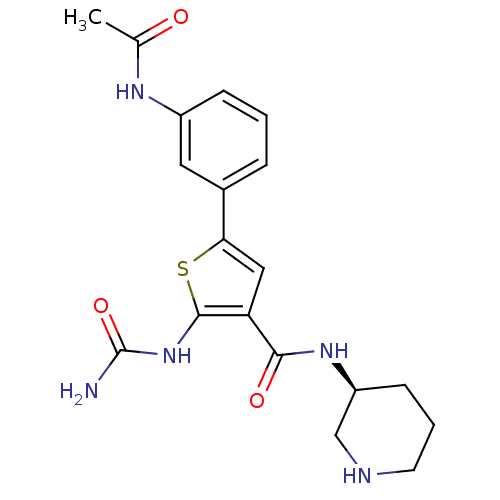
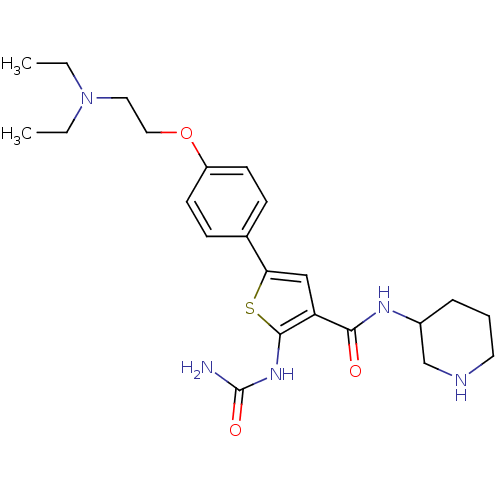
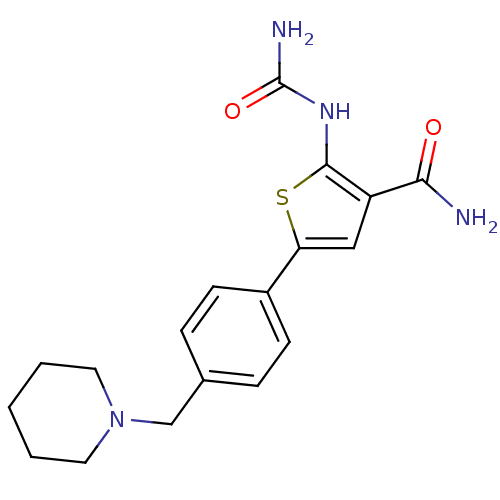
Affinity DataIC50: 2.40nMAssay Description:Inhibition of SMO-mediated hedgehog signalling pathway in human HPEM cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human PARP2 (2 to 583 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by strep...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of human TNKS1 (1001 to 1327 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by s...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of full length human PARP1 expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by streptavidin-...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human PARP2 (2 to 583 residues) expressed in Baculovirus infected Sf9 insect cells using activated DNA as substrate after 1 hr by strep...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of PARP3 (unknown origin) using activated DNA as substrate after 1 hr by streptavidin-horseradish peroxidase-based luminescence assayMore data for this Ligand-Target Pair



 3D Structure (crystal)
3D Structure (crystal)