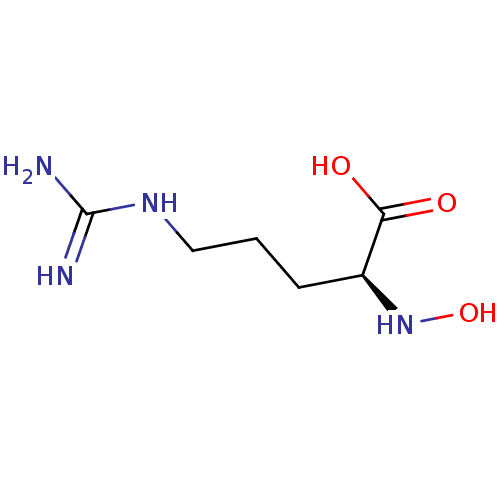
Affinity DataKi: 210nMAssay Description:Inhibition of bovine intestinal alkaline phosphataseMore data for this Ligand-Target Pair
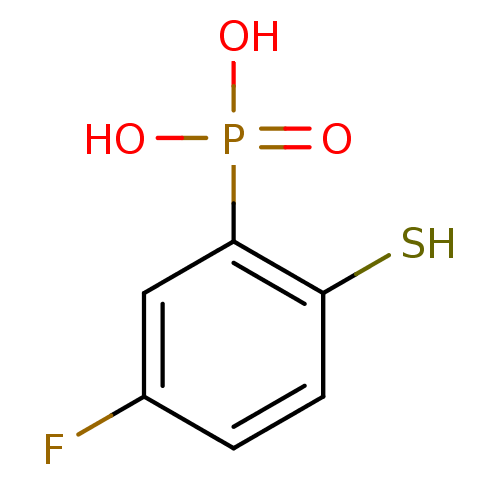
Affinity DataKi: 250nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
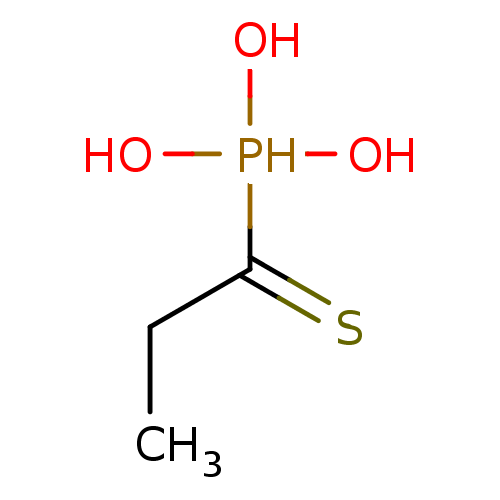
Affinity DataKi: 400nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
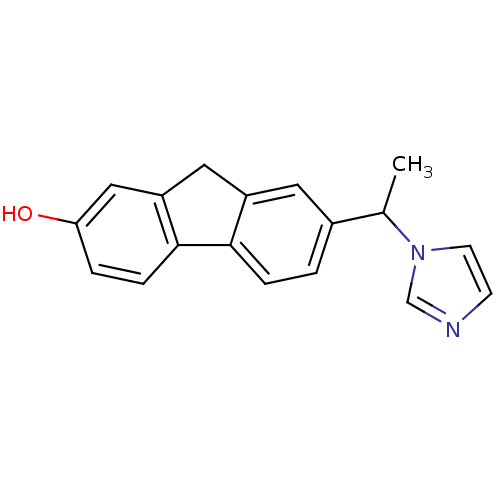
Affinity DataKi: 400nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 1.80E+3nMpH: 8.5Assay Description:Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &...More data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMpH: 8.5Assay Description:Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &...More data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 3.80E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 8.40E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 9.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 9.00E+3nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 1.20E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 1.80E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 2.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 3.20E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: 4.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >5.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >8.00E+4nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of Aeromonas hydrophila cphAMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in presence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetMetallo-beta-lactamase L1 type 3(Stenotrophomonas maltophilia)
University of Li£ge
Curated by ChEMBL
University of Li£ge
Curated by ChEMBL
Affinity DataKi: >2.50E+5nMAssay Description:Inhibition of Stenotrophomonas maltophilia beta lactamase L1 in absence of Zn+ chelatorMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University
Curated by ChEMBL
Saarland University
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of CYP17 (unknown origin)More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University
Curated by ChEMBL
Saarland University
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductaseMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University
Curated by ChEMBL
Saarland University
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductaseMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University
Curated by ChEMBL
Saarland University
Curated by ChEMBL
Affinity DataIC50: 99nMAssay Description:Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductaseMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Saarland University
Curated by ChEMBL
Saarland University
Curated by ChEMBL
Affinity DataIC50: 112nMAssay Description:Inhibition of human CYP17 expressed in Escherichia coli coexpressed with cytochrome P450 reductaseMore data for this Ligand-Target Pair

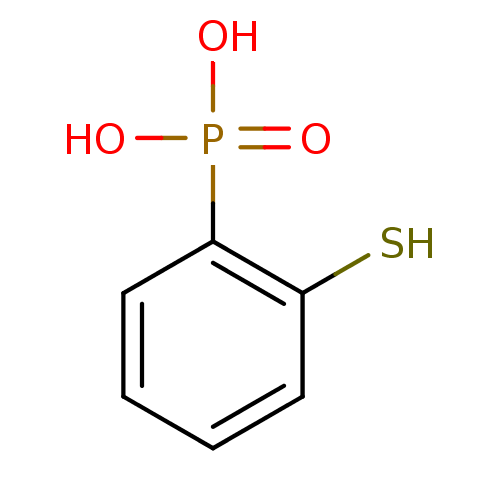

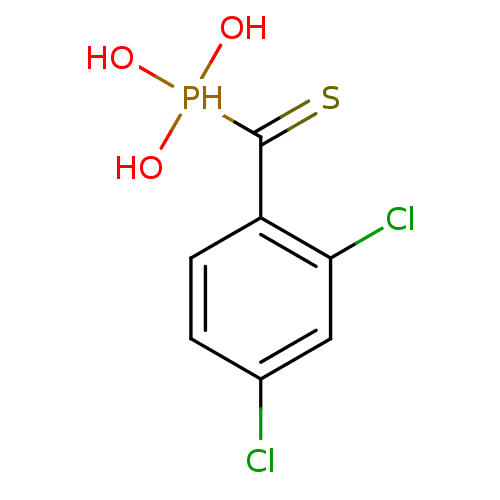

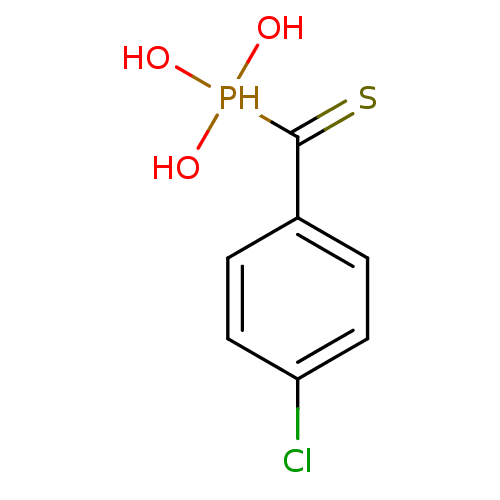
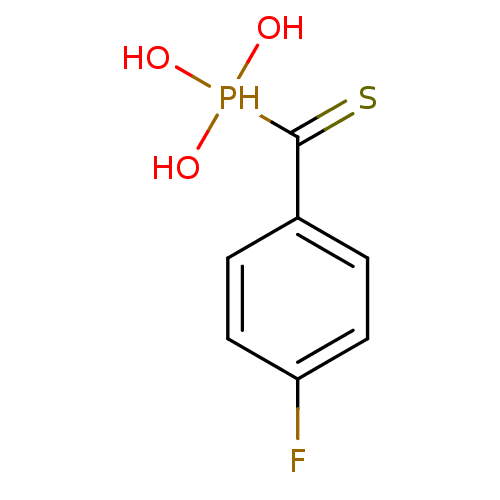
 3D Structure (crystal)
3D Structure (crystal)