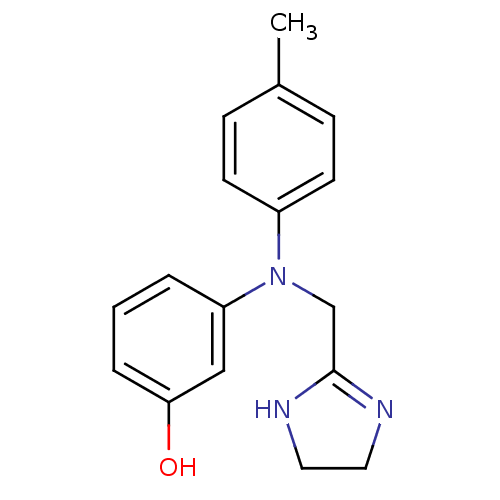
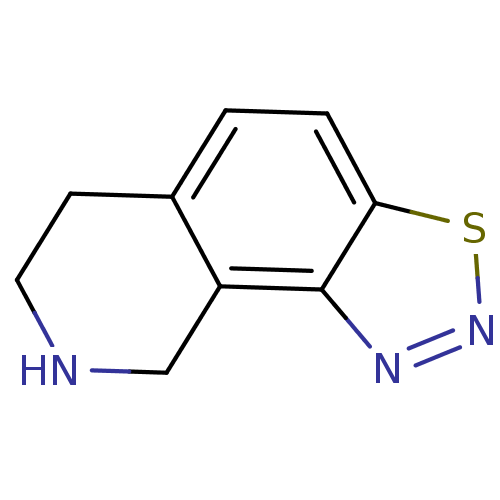
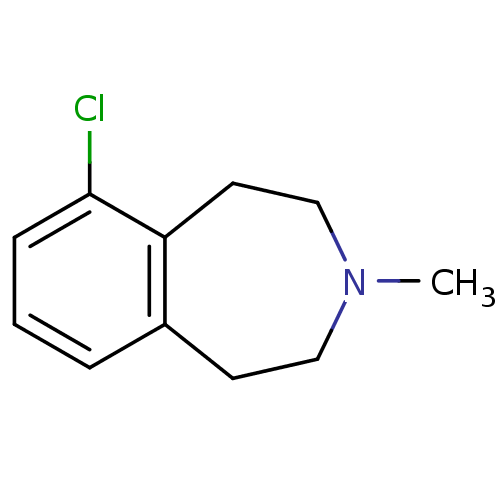
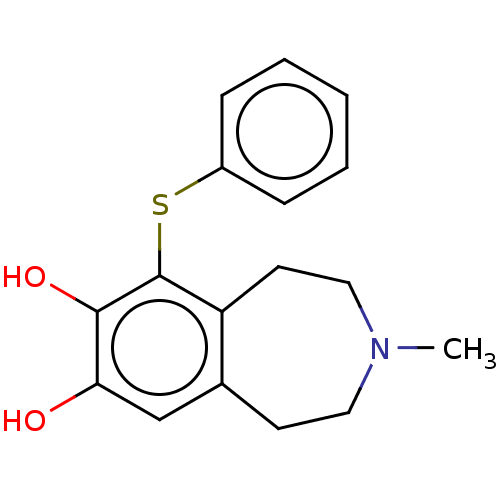
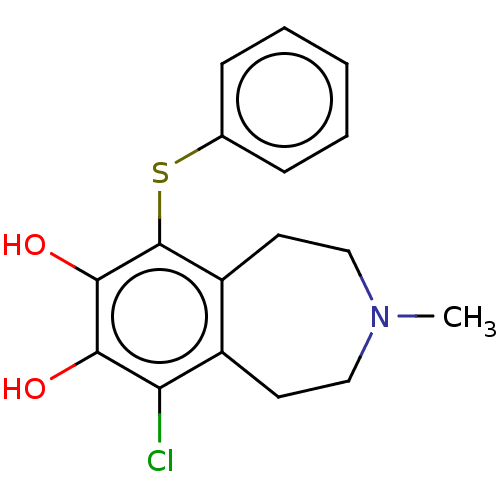
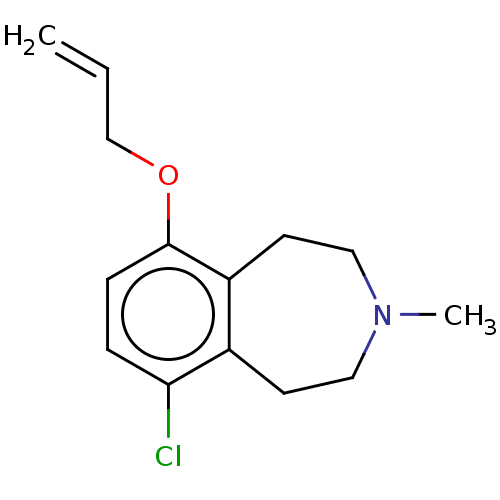
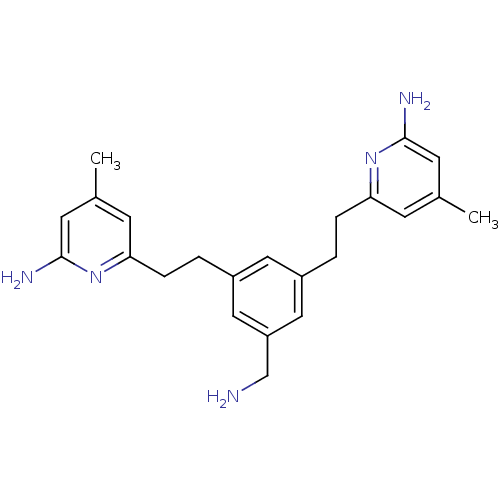
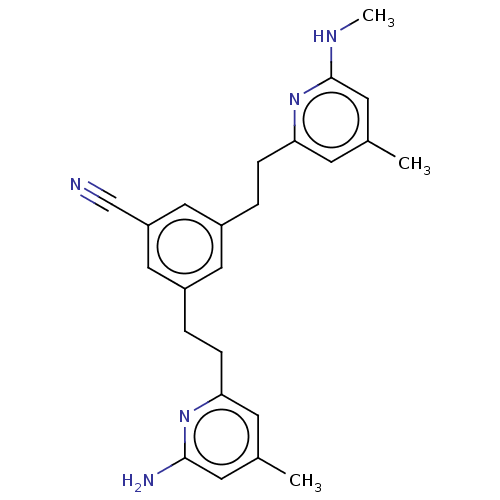
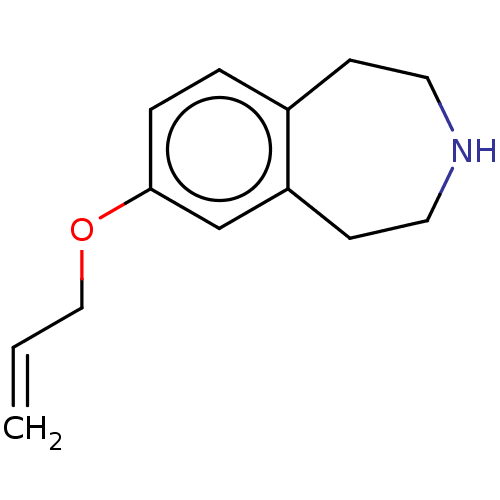
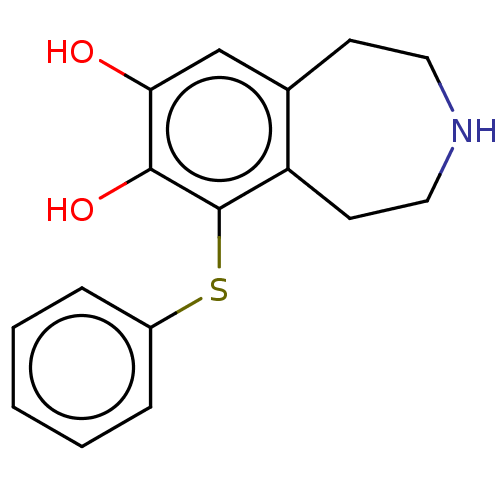
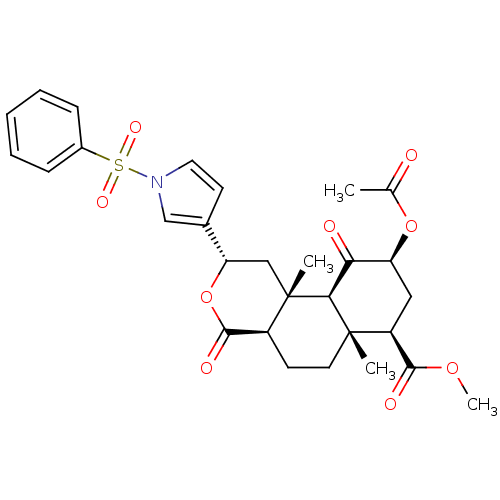
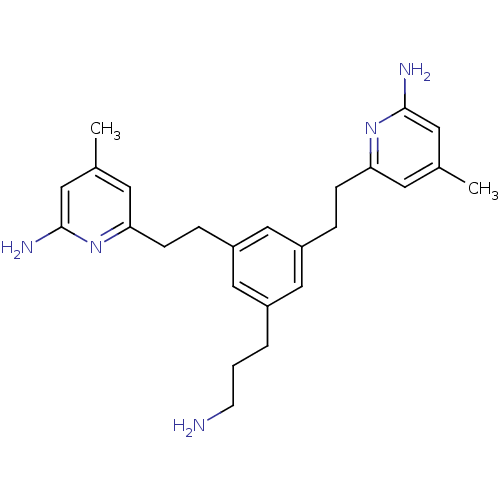
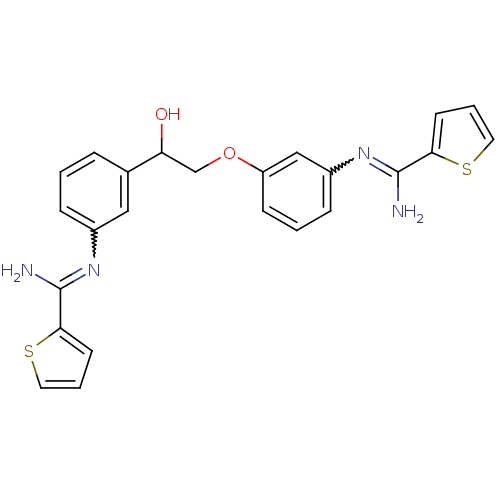
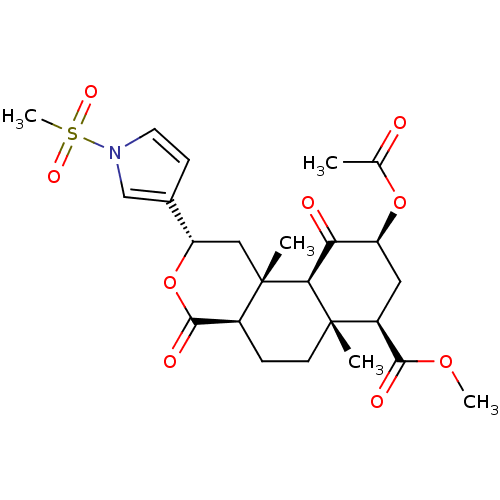
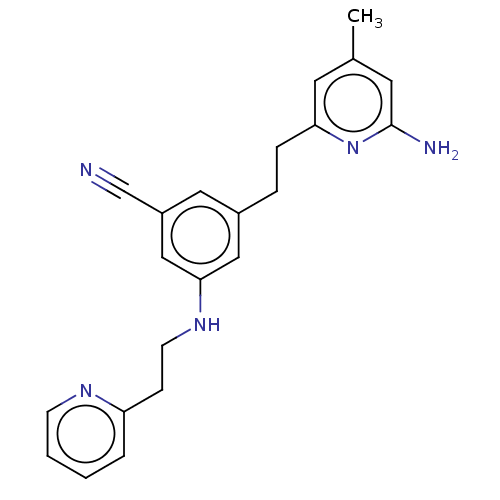
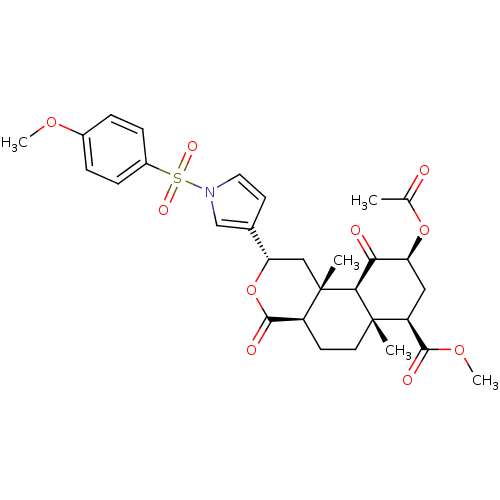
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
Smith Kline& French Laboratories
Curated by ChEMBL
Smith Kline& French Laboratories
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Binding affinity for phenylethanolamine N-methyl-transferase was determined.More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from three separate experiments using 10 i...More data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from three separate experiments using 10 i...More data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Bos taurus (bovine))
Smith Kline& French Laboratories
Curated by ChEMBL
Smith Kline& French Laboratories
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Binding affinity for phenylethanolamine N-methyl-transferase was determined.More data for this Ligand-Target Pair
Affinity DataKi: 17nMAssay Description:Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from three separate experiments using 10 i...More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from four to five separate experiments usi...More data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 118nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 232nMAssay Description:Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in preparations in human platelet membranes from three separate experi...More data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibitory activity against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
Affinity DataKi: 338nMAssay Description:Binding affinity against alpha-2-adrenergic receptor using 10 nM [3H]yohimbine in human platelet membranes from three separate experiments using 10 i...More data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Displacement of [125I]IOXY from human kappa opioid receptorMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 840nMAssay Description:Displacement of [125I]IOXY from human kappa opioid receptorMore data for this Ligand-Target Pair
TargetNitric oxide synthase, brain(Rattus norvegicus (rat))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.27E+3nMAssay Description:Inhibition of rat nNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.56E+3nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
Affinity DataKi: 1.62E+3nMAssay Description:Displacement of [125I]IOXY from human kappa opioid receptorMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 4.95E+3nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 5.20E+3nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 6.31E+3nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 7.10E+3nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
Affinity DataKi: 8.53E+3nMAssay Description:Displacement of [125I]IOXY from human kappa opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human mu opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human mu opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human delta opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human mu opioid receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]IOXY from human mu opioid receptorMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.01E+4nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.17E+4nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.18E+4nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.21E+4nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.28E+4nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 1.44E+4nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, inducible(Mus musculus (mouse))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 3.25E+4nMAssay Description:Inhibition of mouse iNOSMore data for this Ligand-Target Pair
TargetNitric oxide synthase, endothelial(Bos taurus (bovine))
University of California
Curated by ChEMBL
University of California
Curated by ChEMBL
Affinity DataKi: 5.27E+4nMAssay Description:Inhibition of bovine eNOSMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Inhibitory concentration against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibitory concentration against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetAdenylate cyclase type 1/Adenylate cyclase type 2/Adenylate cyclase type 3/Adenylate cyclase type 4/Adenylate cyclase type 5/Adenylate cyclase type 6/Adenylate cyclase type 8/Adenylyl cyclase 7(Rattus norvegicus)
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibitory concentration against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibitory concentration against Dopamine sensitive adenylate cyclase in ratsMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase transforming protein Abl(Abelson murine leukemia virus)
Smith Kline& French Laboratories
Curated by ChEMBL
Smith Kline& French Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of p60v-abl tyrosine kinase from Abelson murine leukemia virusMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)