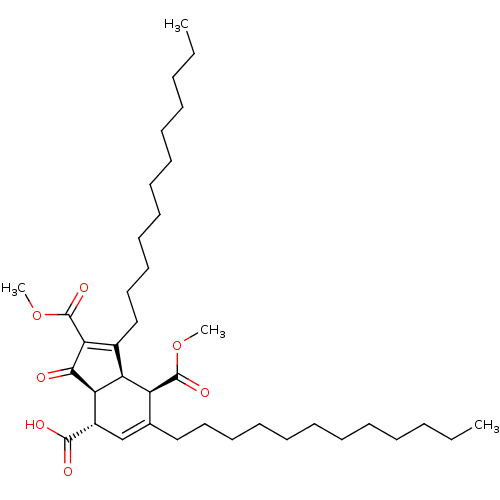
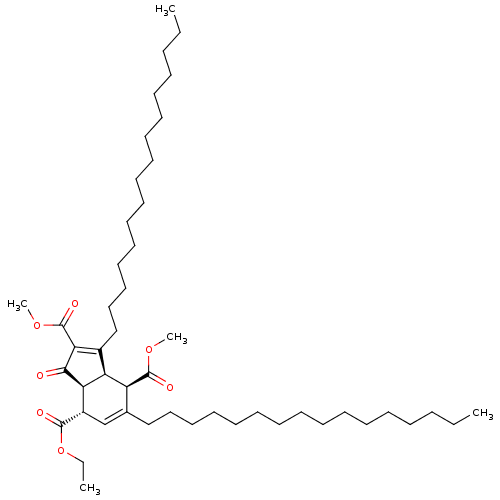
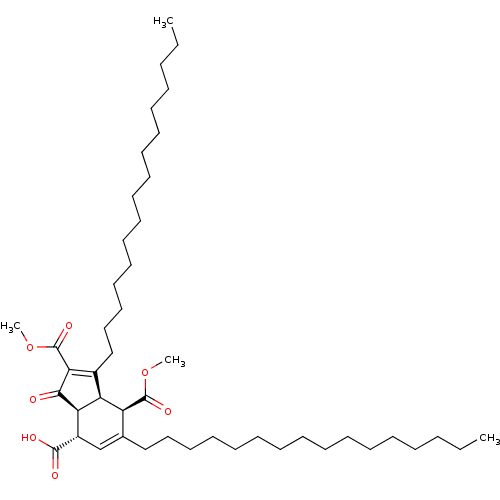
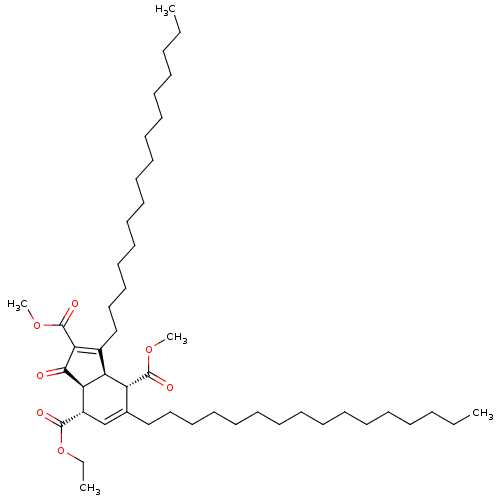
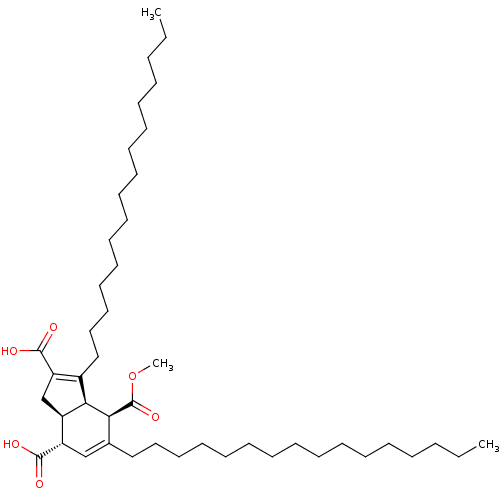
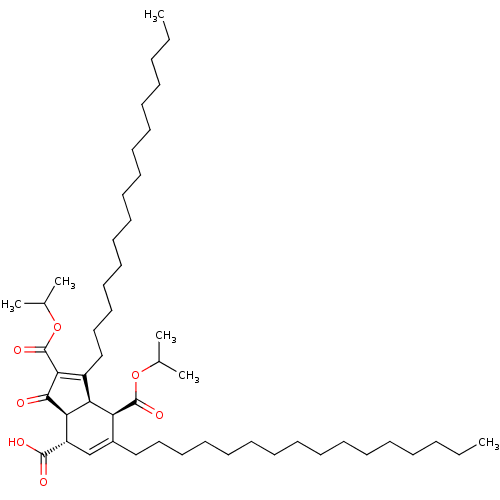
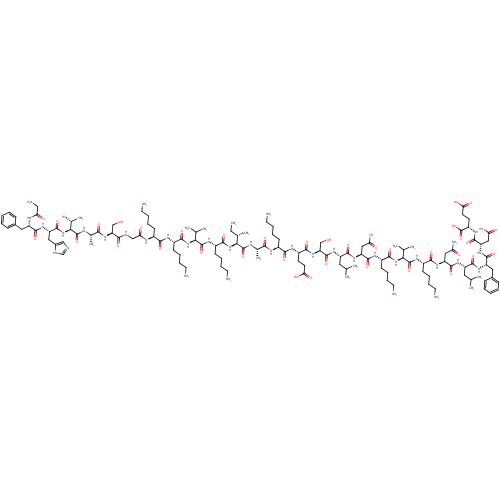
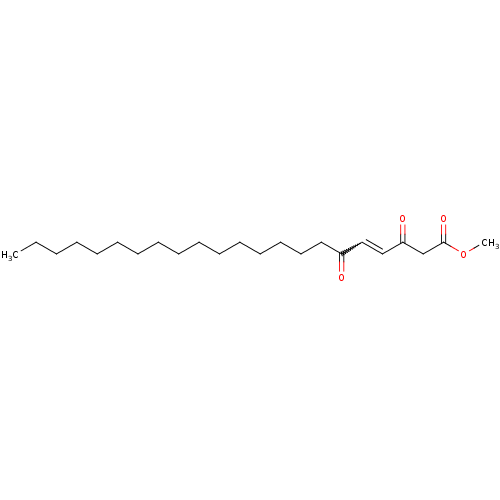
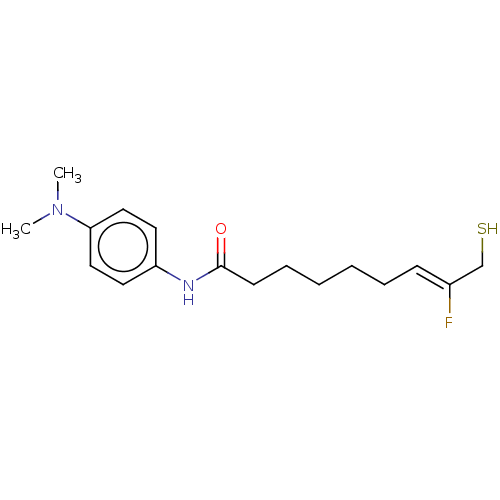
Affinity DataKi: 228nMAssay Description:Non-competitive inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEP...More data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataKi: 750nMAssay Description:Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p...More data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p...More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 3.75E+3nMAssay Description:Inhibition constant against DNA polymerase alpha non competitively on dNTP substrateMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p...More data for this Ligand-Target Pair
Affinity DataKi: 4.70E+3nMAssay Description:Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer doseMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p...More data for this Ligand-Target Pair
Affinity DataKi: 6.60E+3nMAssay Description:Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of nucleotide substrate c...More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 7.84E+3nMAssay Description:Inhibition constant against DNA polymerase alpha non competitively on dNTP substrateMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p...More data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 1.12E+4nMAssay Description:Inhibition constant against DNA polymerase alpha non competitively on dNTP substrateMore data for this Ligand-Target Pair
Affinity DataKi: 1.28E+4nMAssay Description:Inhibition constant of the compound was determined at a concentration of 5 microM on rat DNA polymerase beta as a function of template primer doseMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 1.38E+4nMAssay Description:Inhibition constant against DNA polymerase alpha competitively on DNA templateMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 1.85E+4nMAssay Description:Inhibition constant against DNA polymerase alpha competitively on DNA templateMore data for this Ligand-Target Pair
Affinity DataKi: 2.01E+4nMAssay Description:Inhibition constant of the compound was determined at a concentration of 10 microM on rat DNA polymerase beta as a function of template primer doseMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataKi: 2.79E+4nMAssay Description:Inhibition constant against DNA polymerase alpha competitively on DNA templateMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataKi: >4.00E+4nMAssay Description:Inhibition of human RAD51 assessed as concentration required for half dissociation of protein/single-stranded DNA complex formation by fluorescence p...More data for this Ligand-Target Pair
Affinity DataIC50: 86nMAssay Description:Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N...More data for this Ligand-Target Pair
Affinity DataIC50: 87nMAssay Description:Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N...More data for this Ligand-Target Pair
Affinity DataIC50: 215nMAssay Description:Inhibition of His-tagged PPM1D (1 to 420 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) pLysS cells using Ac-VEPPLS(P)QETFSDLW-N...More data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of N-terminal histidine-tagged PPM1Dc after 10 mins using BIOMOL GREEN assayMore data for this Ligand-Target Pair
Affinity DataIC50: 510nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assayMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 1.05E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extract by fluorescence assayMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded poly-(d-epsilonA) complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of DNA polymerase betaMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded DNA complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of DNA polymerase betaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of DNA polymerase betaMore data for this Ligand-Target Pair
TargetDNA nucleotidylexotransferase(Homo sapiens (Human))
The University of Manchester
Curated by ChEMBL
The University of Manchester
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human TdTMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded poly-(d-epsilonA) complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
TargetDNA nucleotidylexotransferase(Homo sapiens (Human))
The University of Manchester
Curated by ChEMBL
The University of Manchester
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibitory activity of the compound against human terminal deoxynucleotidyltransferaseMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded oligo(AGT)12 complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of DNA polymerase betaMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded DNA complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
TargetDNA polymerase alpha catalytic subunit(Homo sapiens (Human))
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Frontier Research Center for Genome & Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of DNA polymerase alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of DNA polymerase betaMore data for this Ligand-Target Pair
TargetDNA repair protein RAD51 homolog 1(Homo sapiens (Human))
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Centre National de la Recherche Scientifique& Universite de Nantes
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human RAD51 assessed as dissociation of protein/single-stranded poly-(d-epsilonA) complex formation by fluorescence polarimetryMore data for this Ligand-Target Pair

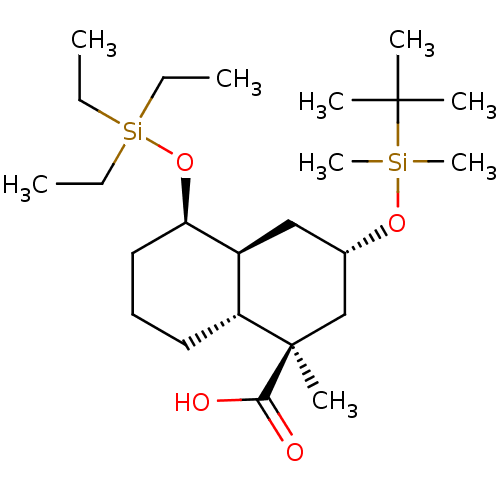
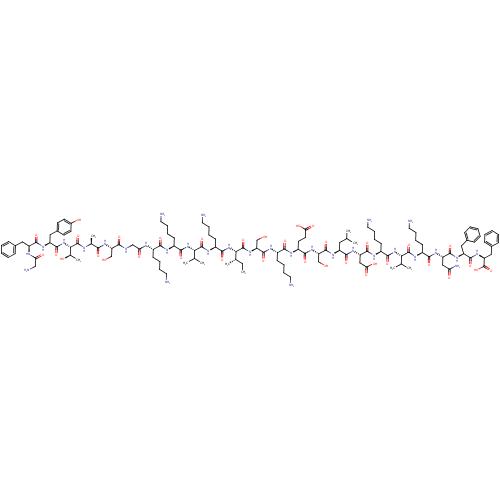
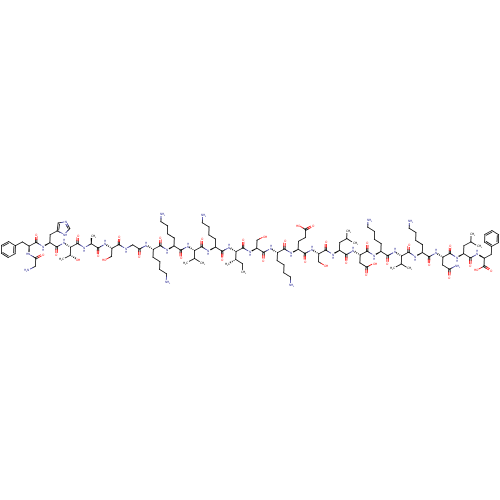
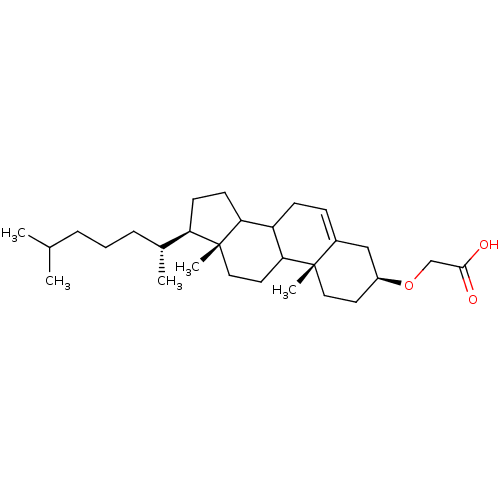

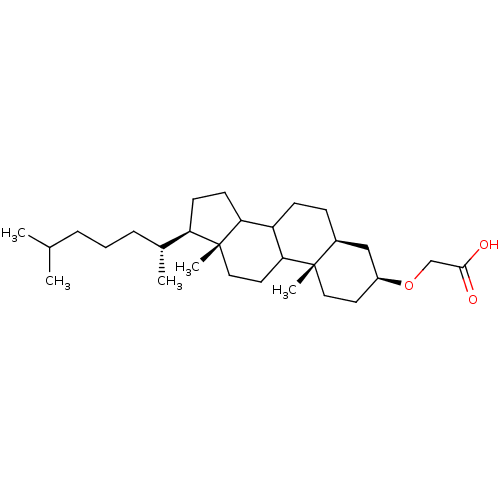
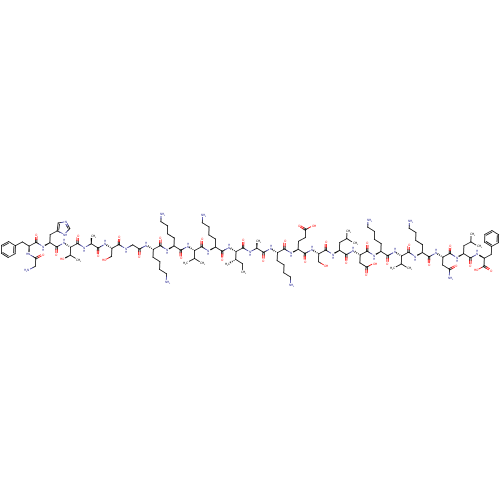
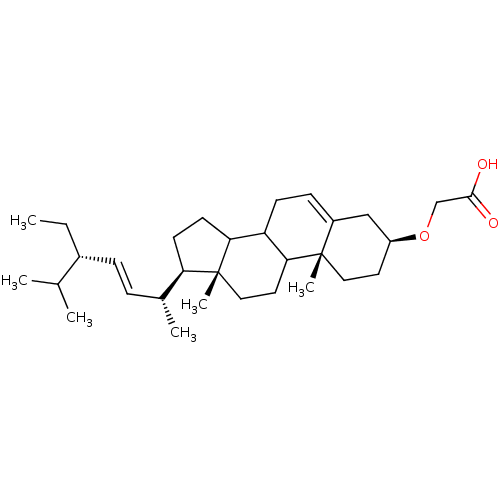
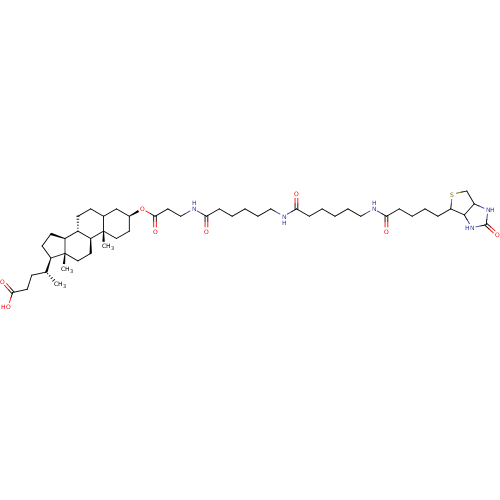
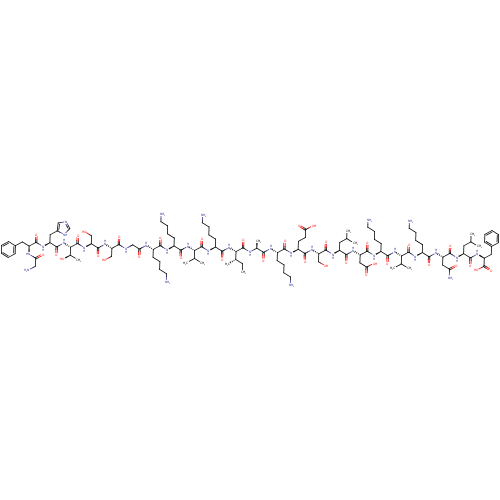
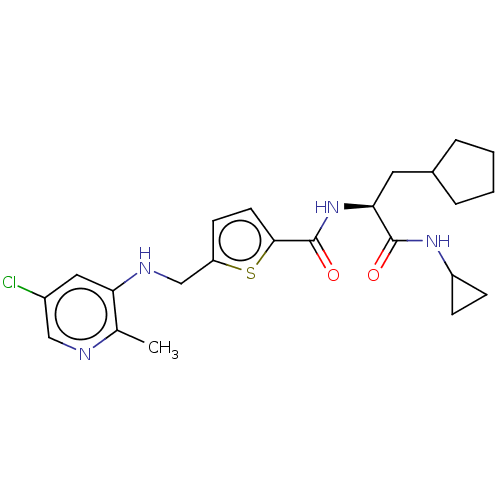
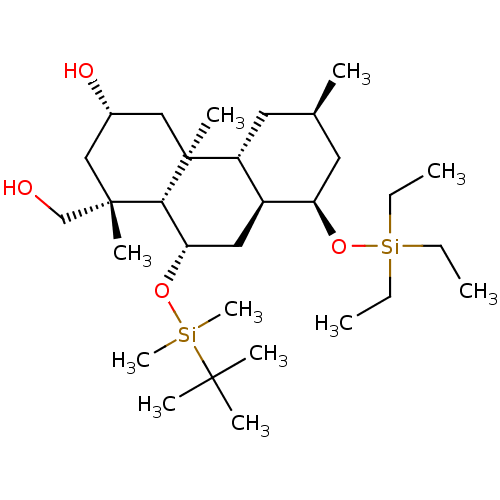
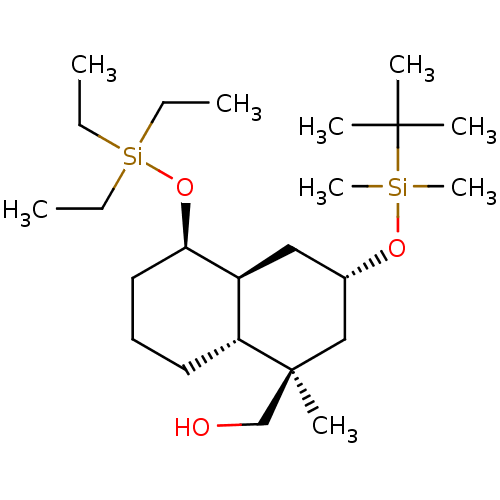
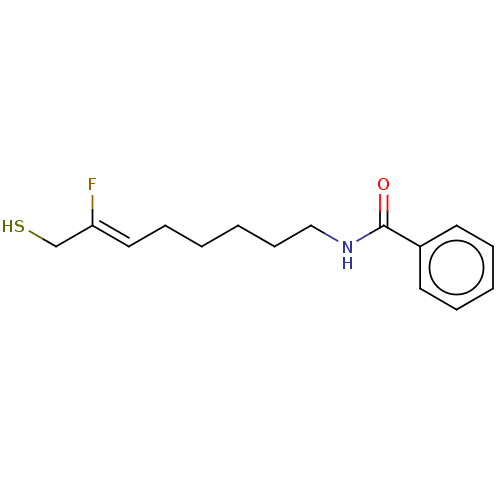
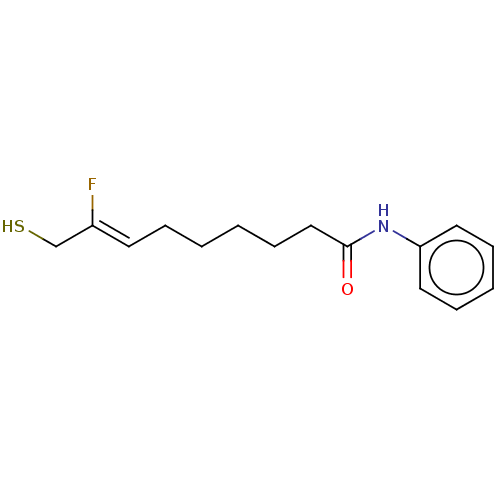
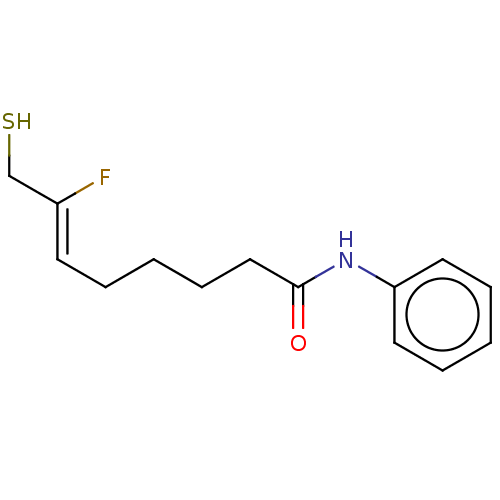

 3D Structure (crystal)
3D Structure (crystal)