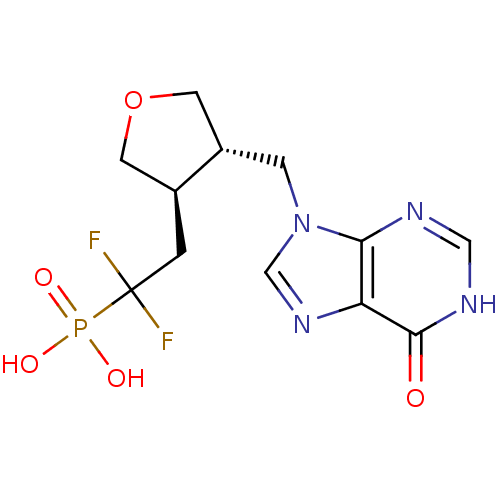
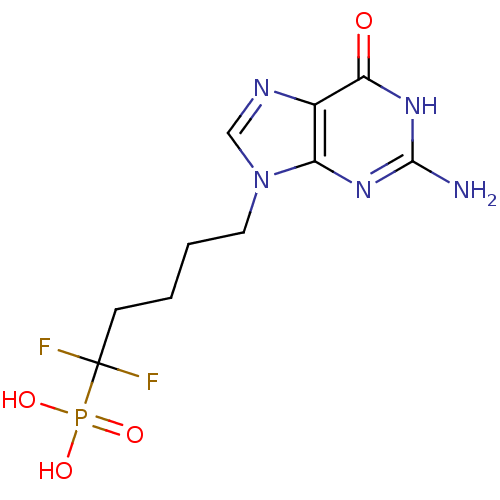
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 8.80nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataKi: 53nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas spMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas spMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas spMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 88nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas spMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 390nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in human erythrocyteMore data for this Ligand-Target Pair
TargetPurine nucleoside phosphorylase(Homo sapiens (Human))
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
Affinity DataIC50: 540nMAssay Description:Compound was evaluated for inhibition of PNP-catalyzed inosine phosphorylation in Cellulomonas spMore data for this Ligand-Target Pair

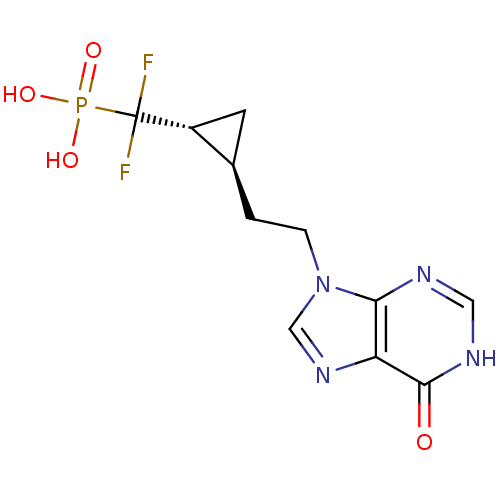
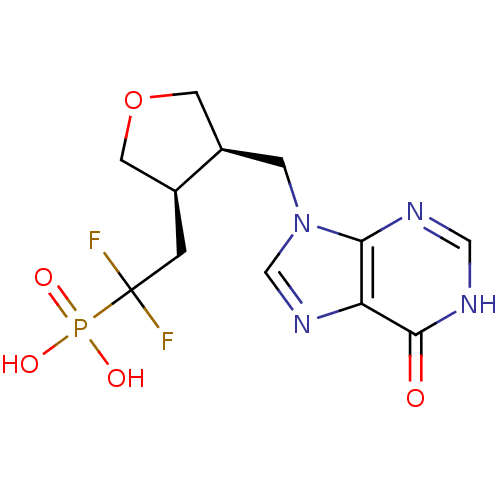
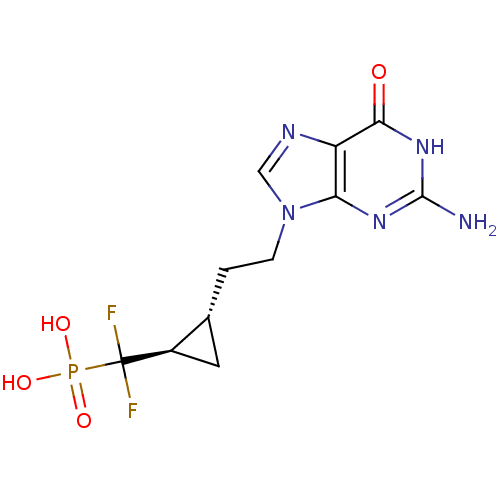
 3D Structure (crystal)
3D Structure (crystal)