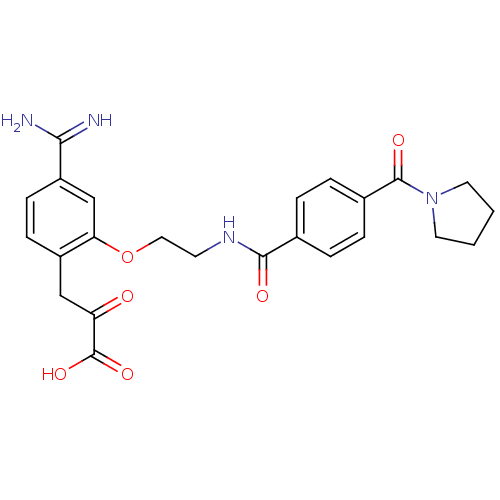
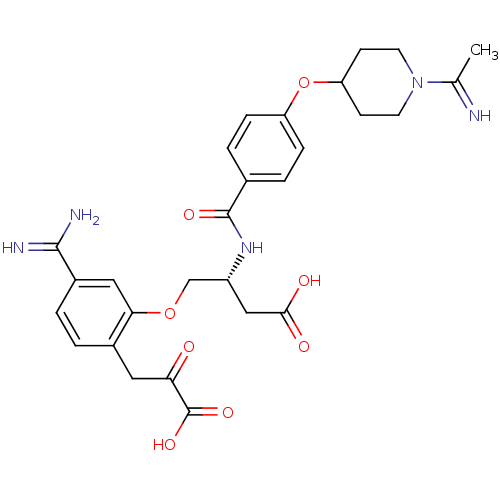
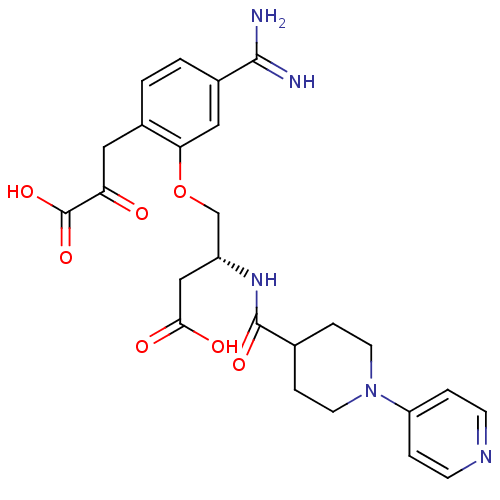
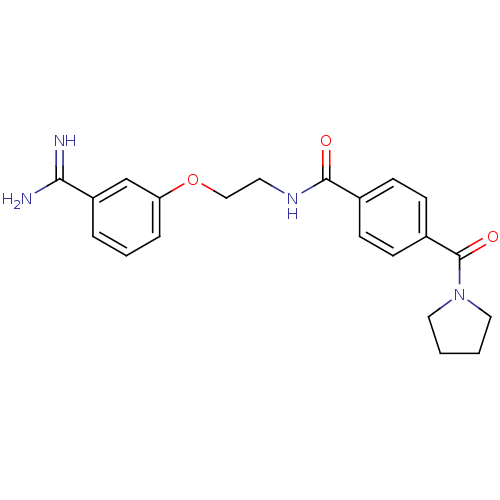
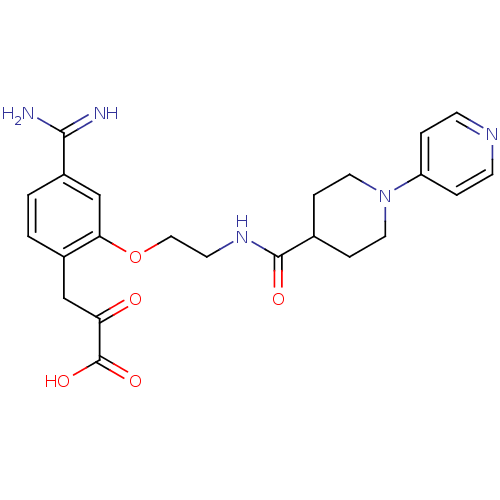
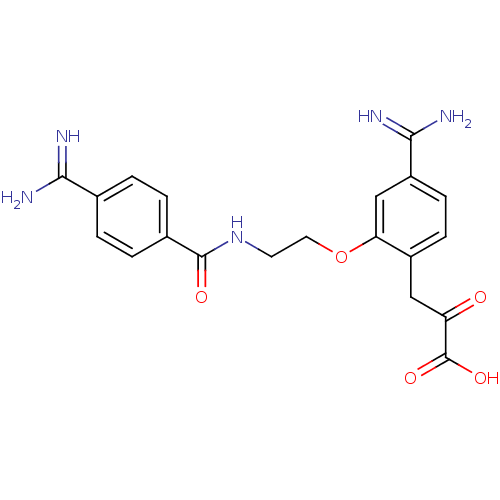
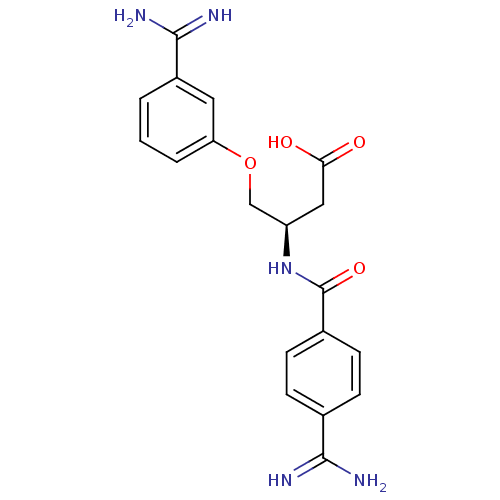
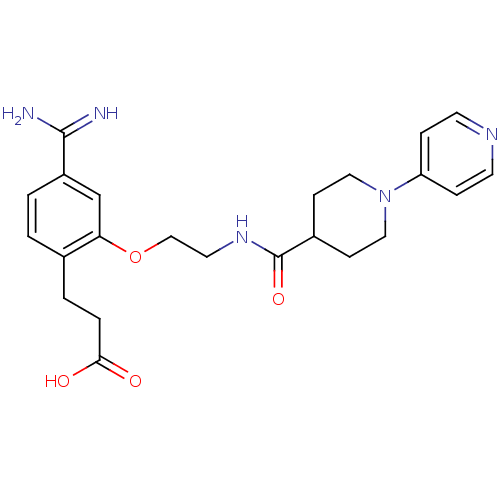
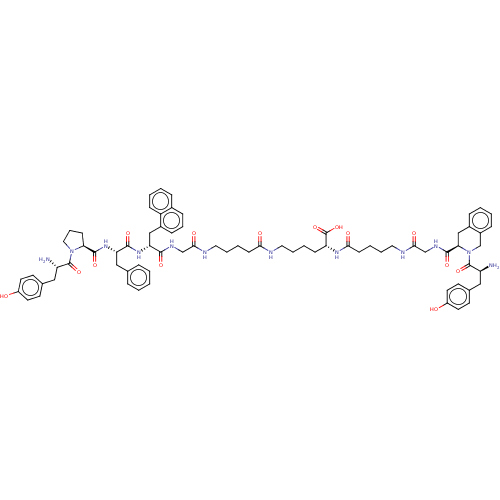
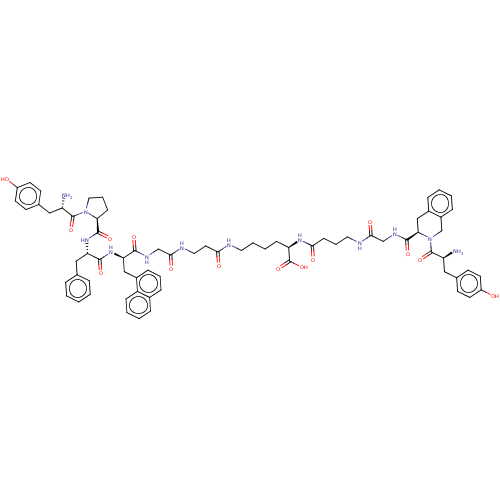
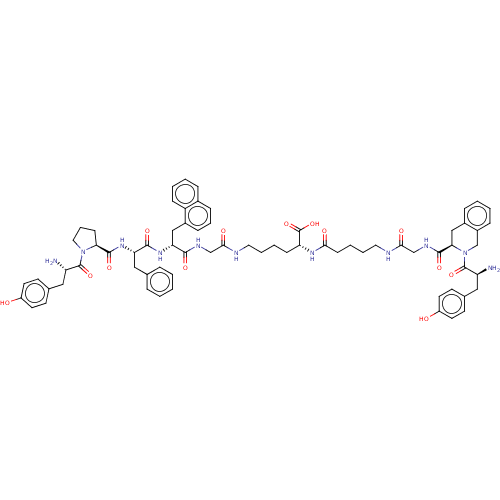
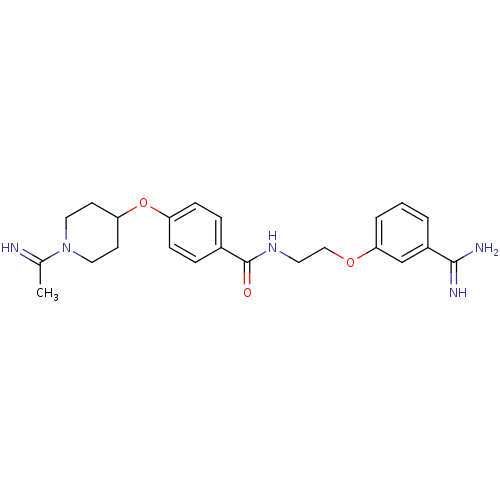
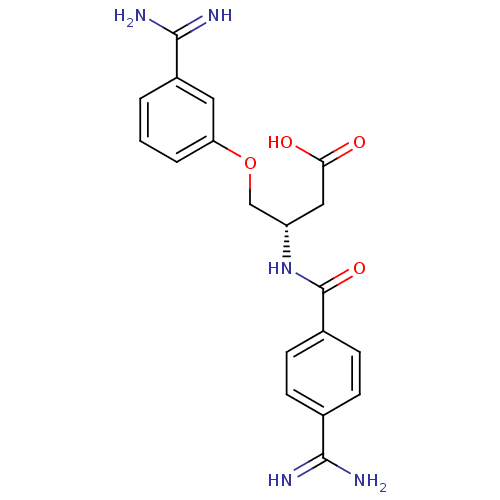
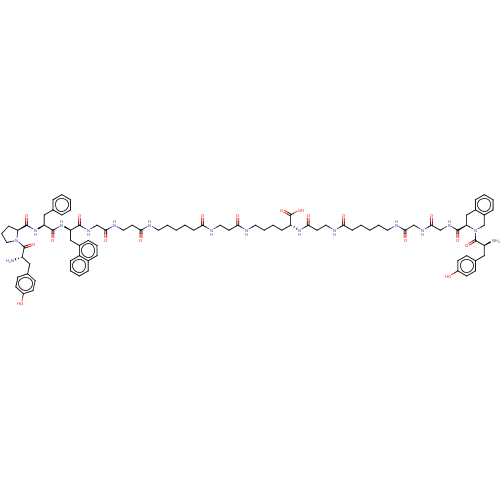
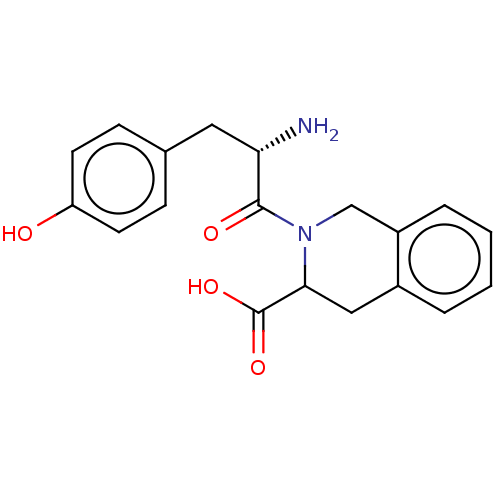
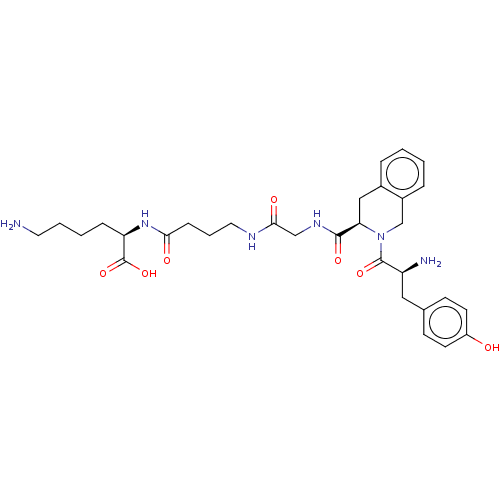
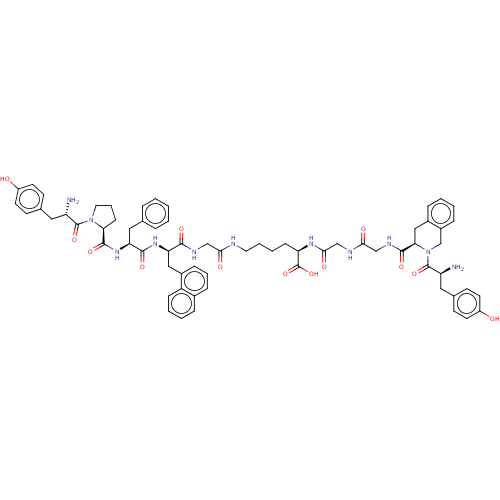
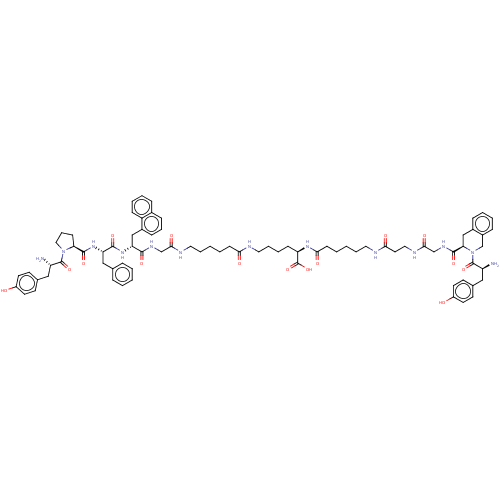
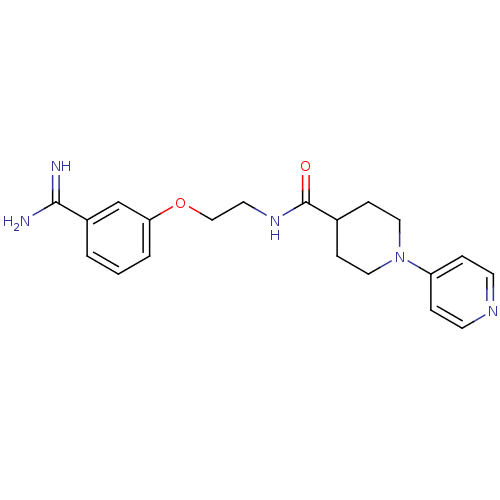
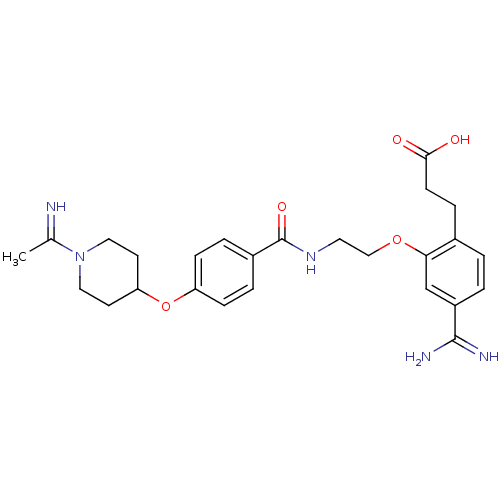
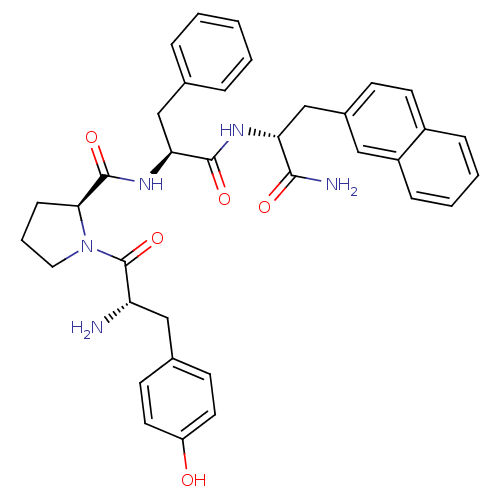
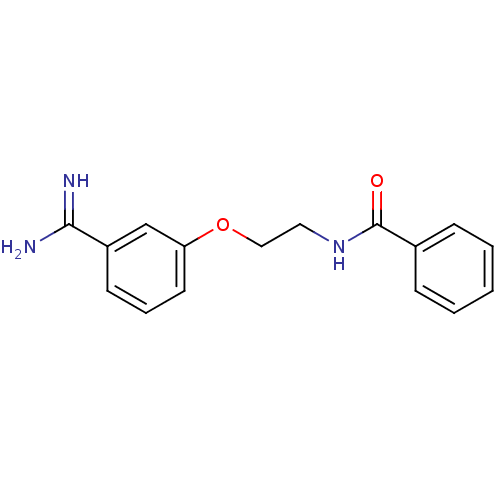
Affinity DataKi: 3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 84nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 98nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 220nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 270nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Displacement of [3H]-diprenorphine from human DOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-diprenorphine from human MOR expressed in CHO cell membranes after 80 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Inhibitory activity against Coagulation factor X (fXa)More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+4nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 7.10E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+5nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataKi: >1.50E+6nMAssay Description:Inhibitory activity against thrombinMore data for this Ligand-Target Pair
Affinity DataIC50: 9.30nMAssay Description:Antagonist activity at human KOR expressed in CHO cell membranes assessed as inhibition of U50488-induced [35S]-GTPgammaS binding preincubated for 5 ...More data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ...More data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ...More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ...More data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:Antagonist activity at human DOR expressed in CHO cell membranes assessed as inhibition of CYM51010-induced [35S]-GTPgammaS binding preincubated for ...More data for this Ligand-Target Pair