Affinity DataKi: 0.0269nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0910nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.131nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Antagonist activity at muscarinic M2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Antagonist activity at muscarinic M1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.276nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.290nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.305nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.310nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.340nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.350nMAssay Description:Antagonist activity at muscarinic M4 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.397nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.545nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
Affinity DataKi: 0.634nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.679nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
Affinity DataKi: 0.730nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 0.765nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.990nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Antagonist activity at muscarinic M5 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.60nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.31nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [3H]N-methyl scopolamine from human cloned muscarinic M3 receptor by dilution methodMore data for this Ligand-Target Pair
Affinity DataKi: 32.4nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
Affinity DataKi: 62.3nMpH: 7.5Assay Description:Assay buffer was 20 mM HEPES, pH 7.5, 10 mM MgCl2, 0.01% BSA, 1 mM DTT, 0.0005% Tween 20, and 2% DMSO. Inactivation kinetic reactions were performed ...More data for this Ligand-Target Pair
TargetMaternal embryonic leucine zipper kinase(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence...More data for this Ligand-Target Pair
Affinity DataIC50: 0.346nMpH: 7.4 T: 2°CAssay Description:Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ...More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMpH: 7.4 T: 2°CAssay Description:Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ...More data for this Ligand-Target Pair
TargetMaternal embryonic leucine zipper kinase(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils.More data for this Ligand-Target Pair
TargetMaternal embryonic leucine zipper kinase(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils.More data for this Ligand-Target Pair
TargetMaternal embryonic leucine zipper kinase(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Binding affinity to MELK (unknown origin) expressed in Escherichia coli by SPR analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils.More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMpH: 7.4 T: 2°CAssay Description:Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMpH: 7.4 T: 2°CAssay Description:Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils.More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMpH: 7.4 T: 2°CAssay Description:Test compounds were solubilized in dimethyl sulfoxide (DMSO) to a stock concentration of 30 mM. Compounds were diluted in DMSO to create an 11-point ...More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy...More data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Agonist activity at adenosine A2A receptor in fMLP-stimulated human neutrophils assessed as inhibition of superoxide production by colorimetric analy...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils.More data for this Ligand-Target Pair


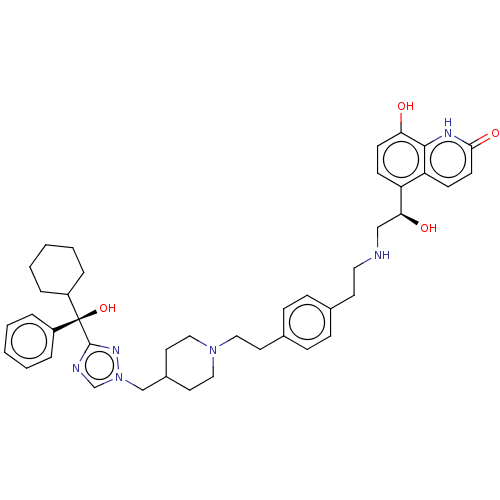
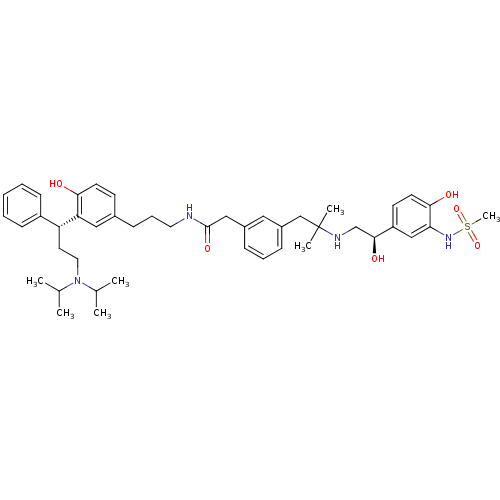
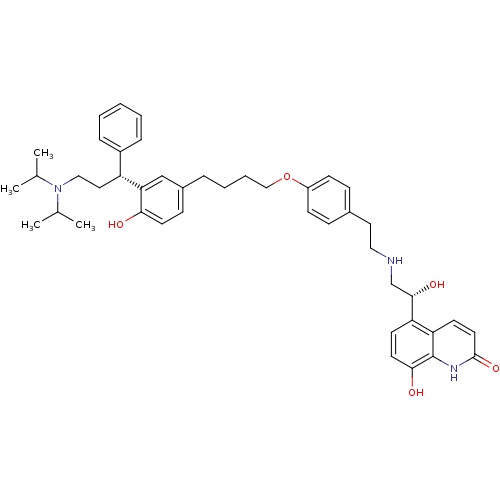
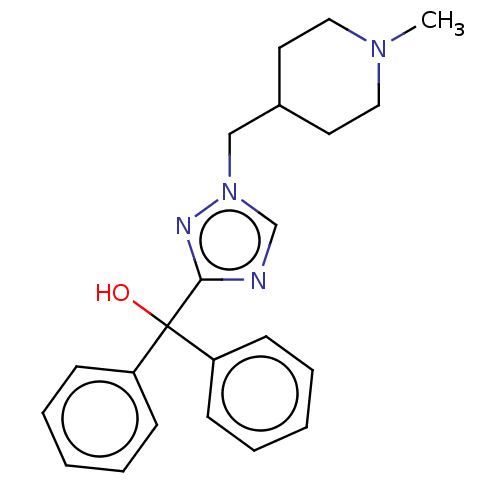

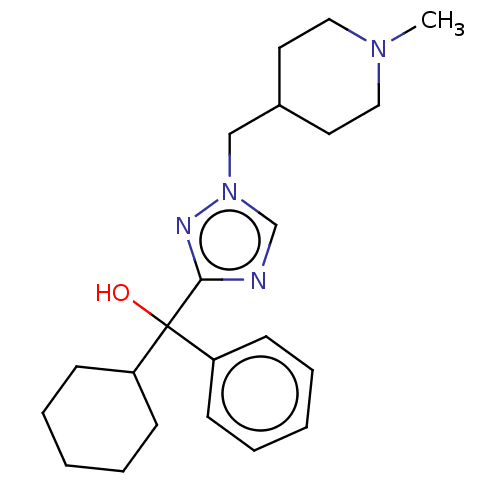
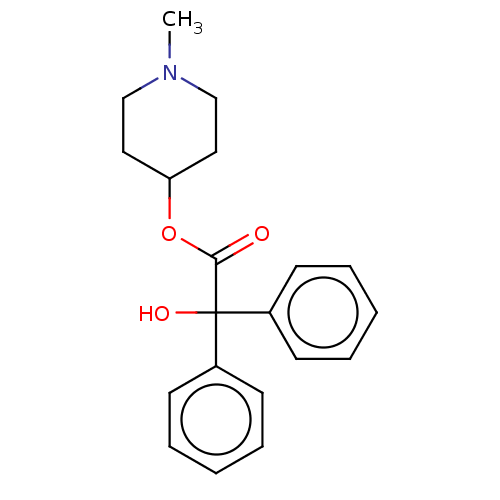
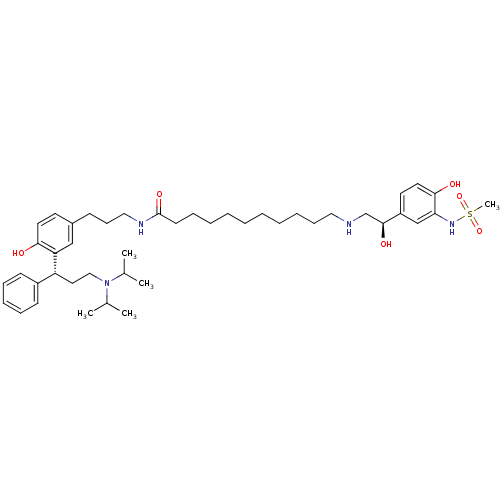

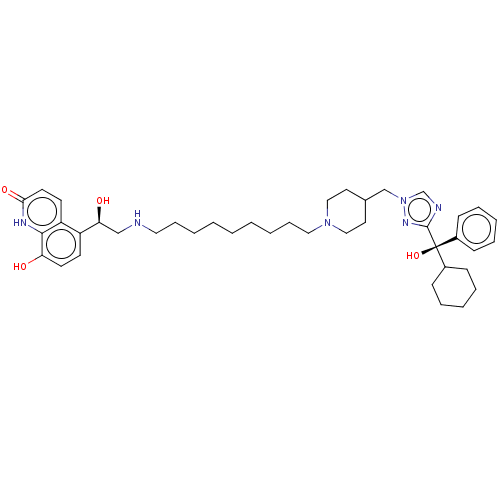

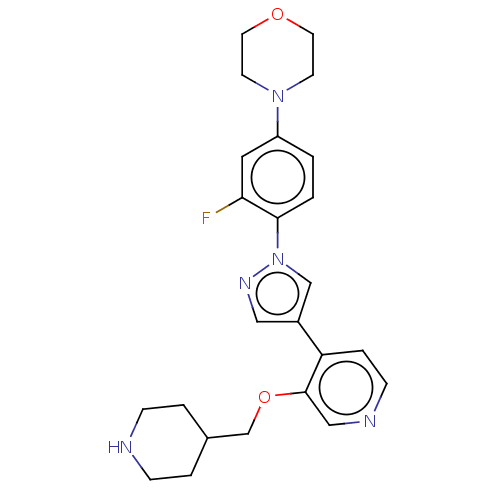
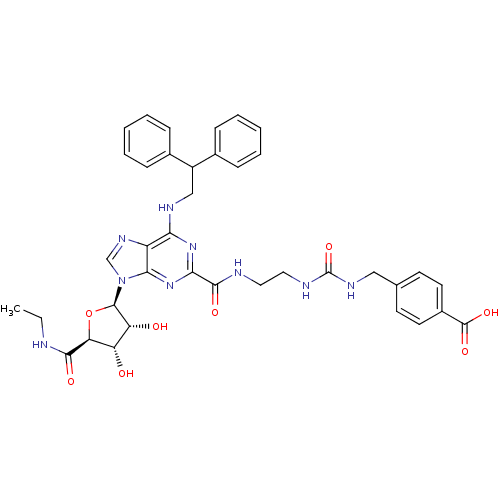

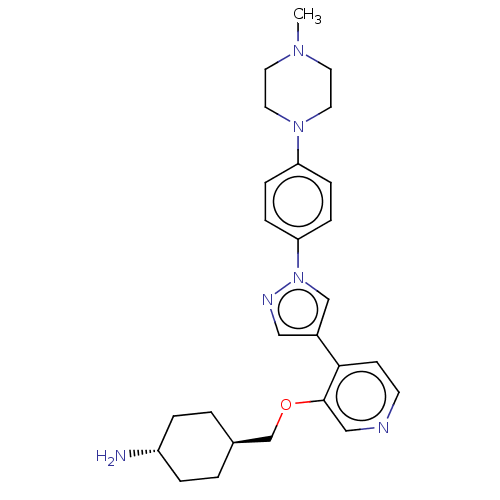


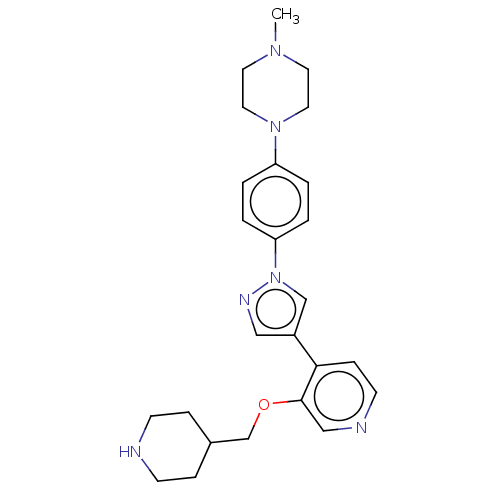
 3D Structure (crystal)
3D Structure (crystal)