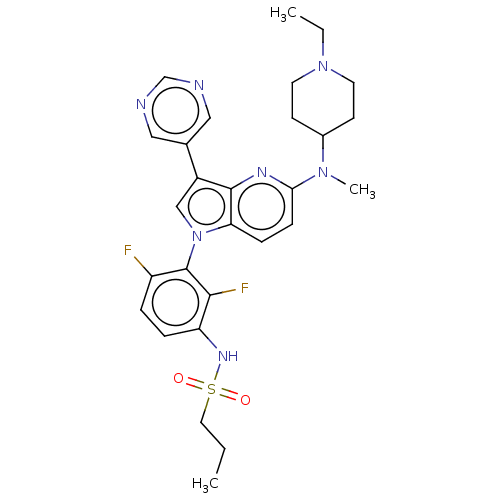
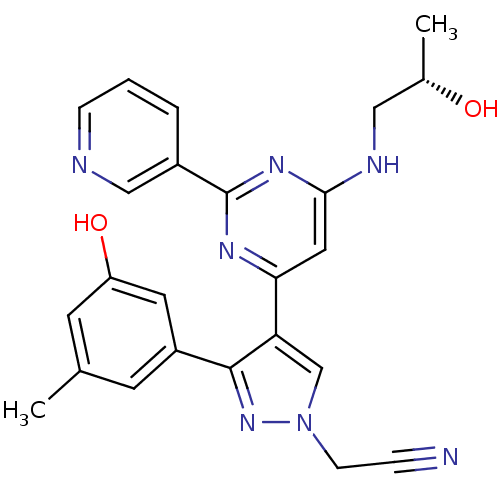
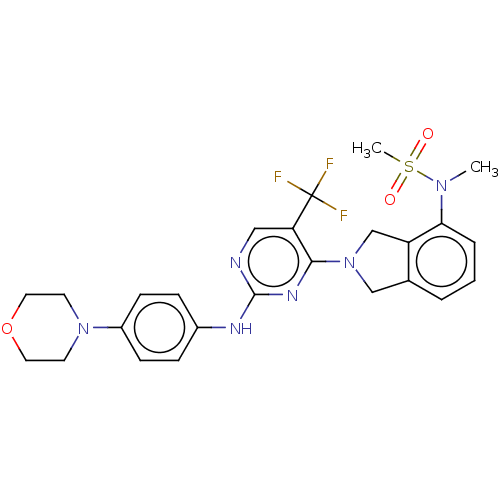
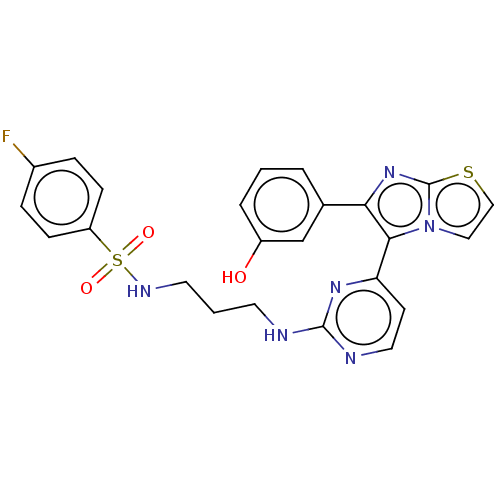
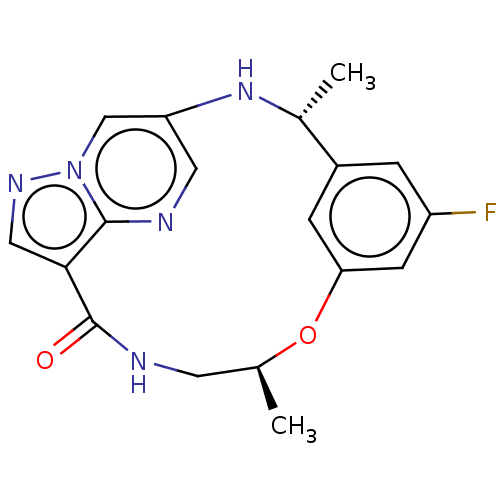
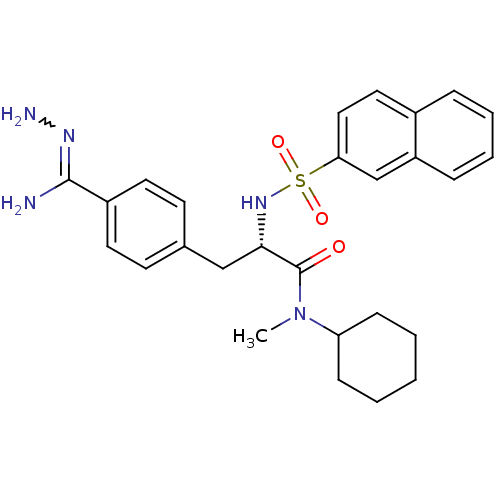
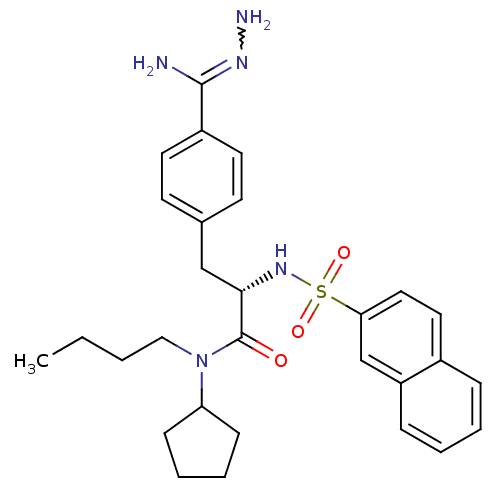
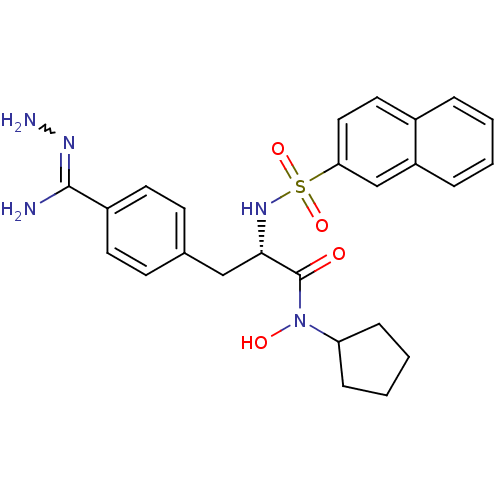
Affinity DataKi: 0.380nMAssay Description:Compound was tested for inhibitory activity against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 93nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 152nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 231nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 247nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 367nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:The compound was tested for inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 1.47E+3nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 3.29E+3nMAssay Description:Compound was tested for inhibitory activity against bovine trypsinMore data for this Ligand-Target Pair
Affinity DataKi: 7.04E+3nMAssay Description:Compound was tested for inhibitory activity against bovine thrombinMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Biotech Research Institute
Curated by ChEMBL
Biotech Research Institute
Curated by ChEMBL
Affinity DataKi: 2.72E+4nMAssay Description:The compound was tested for inhibitory activity against tissue plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 4.79E+4nMAssay Description:The compound was tested for inhibitory activity against human plasminMore data for this Ligand-Target Pair
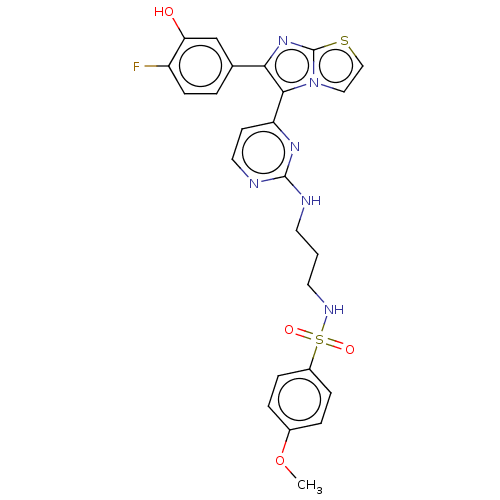
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.0400nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.0400nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.0700nMAssay Description:Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase ROS(Homo sapiens (Human))
Kyung Hee University
Curated by ChEMBL
Kyung Hee University
Curated by ChEMBL
Affinity DataIC50: 0.0700nMAssay Description:Inhibition of ROS1 by HotSpot assay relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.270nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.370nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Korea University of Science and Technology
Curated by ChEMBL
Korea University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of c-Met kinase (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.400nMAssay Description:Inhibition of BRAF (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.5nMAssay Description:Inhibition of BRAF V600E mutant (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human AXL using EAIYAAPFAKKK as substrate by [gamma-33P]-ATP assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea University of Science and Technology
Curated by ChEMBL
Korea University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of ALK (unknown origin) incubated for 20 mins followed by [33P]ATP addition measured after 120 mins by HotSpot assayMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea University of Science and Technology
Curated by ChEMBL
Korea University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Inhibition of ALK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase ROS(Homo sapiens (Human))
Kyung Hee University
Curated by ChEMBL
Kyung Hee University
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of ROS1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.75nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:Inhibition of N-terminal His-tagged recombinant human AXL (473 to end amino acids) expressed by baculovirus in Sf9 cells using axltide substrate and ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
Affinity DataIC50: 0.980nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 0.980nMAssay Description:Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Homo sapiens (Human))
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
KOREA INSTITUTE OF SCIENCE AND TECHNOLOGY
US Patent
Affinity DataIC50: 1nMAssay Description:Inhibition of human BRAF V600E mutant using myelin basic protein as substrate in presence of [gamma-33P]ATP incubated for 40 mins by scintillation co...More data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Korea University of Science and Technology
Curated by ChEMBL
Korea University of Science and Technology
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Reaction biology kinase hotspot service (http://www.reactionbiology.com) was used to measure IC50. In a final reaction volume of 25 μL, kinase (...More data for this Ligand-Target Pair


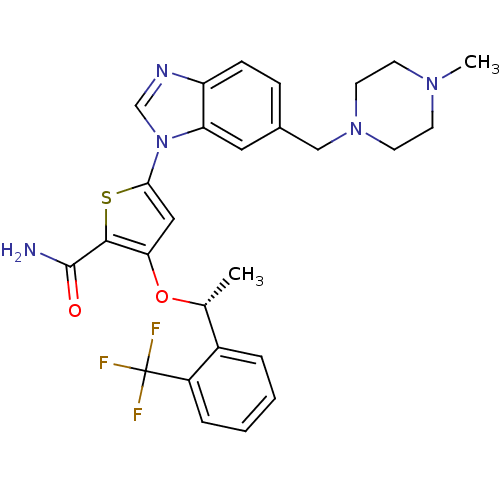
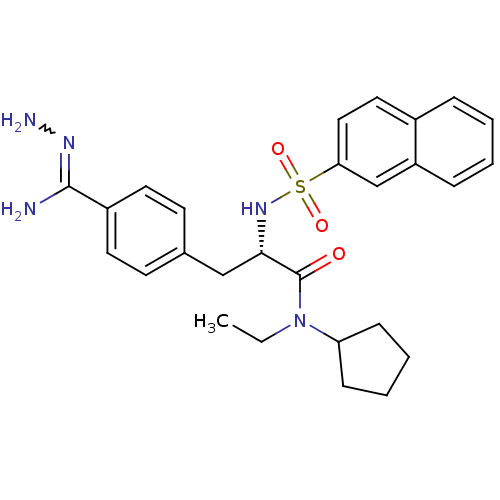

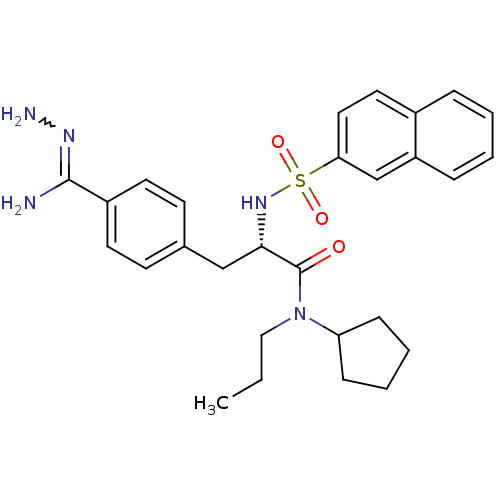




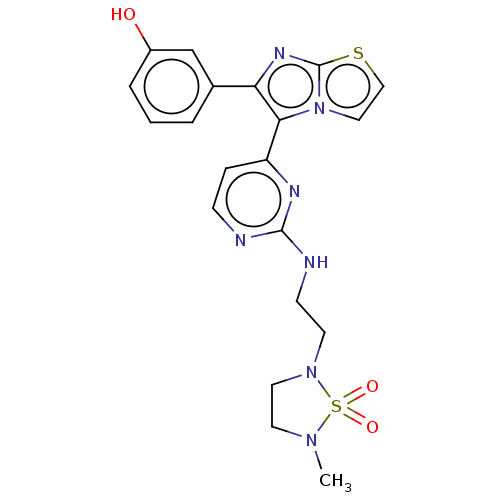

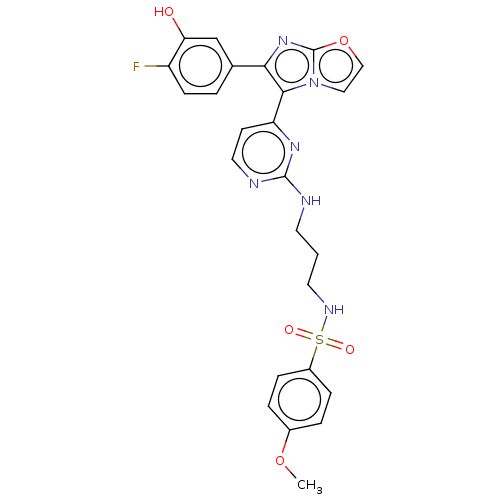

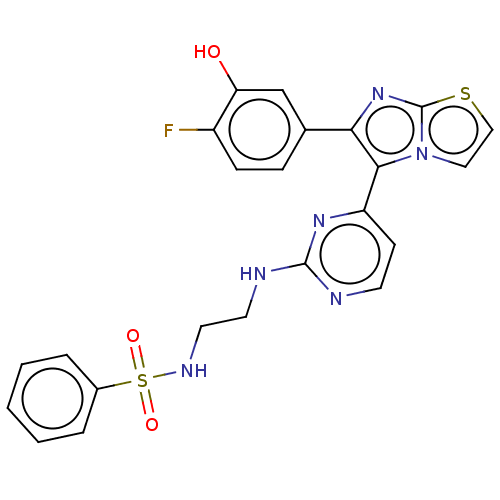

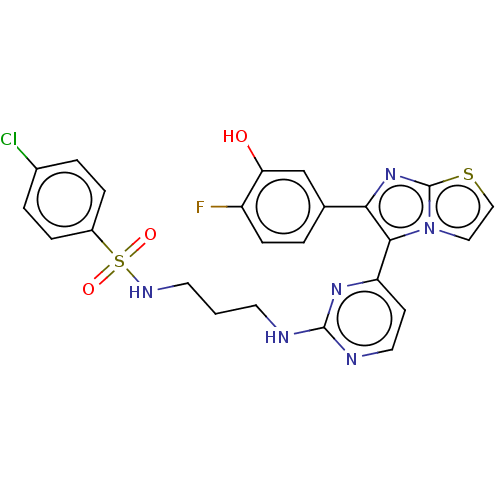



 3D Structure (crystal)
3D Structure (crystal)