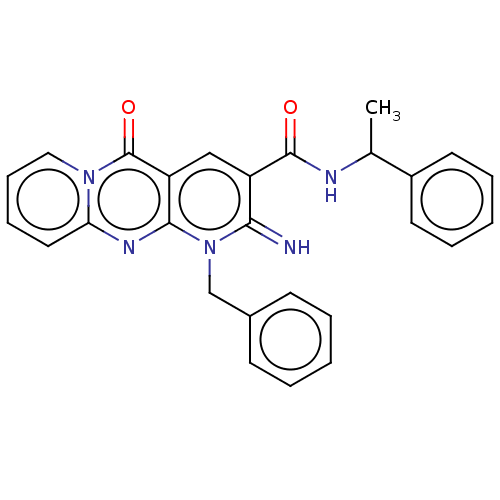
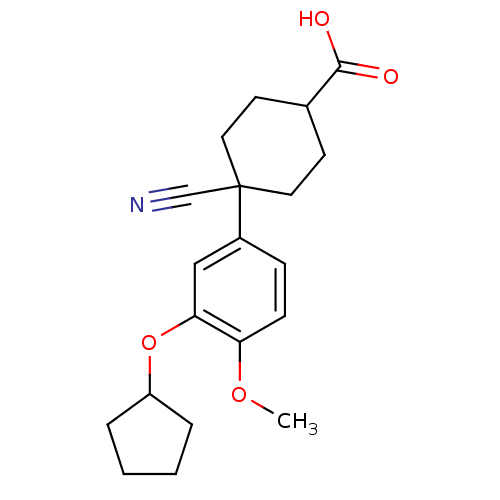
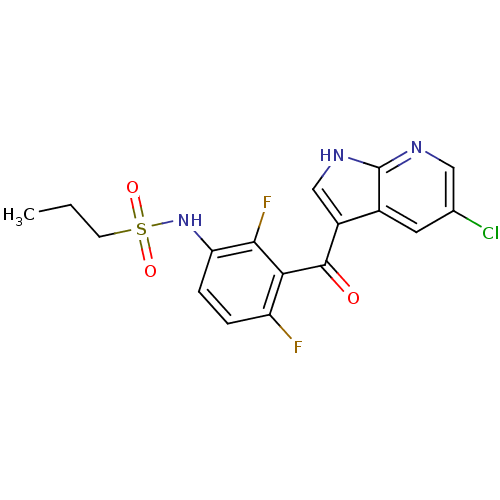
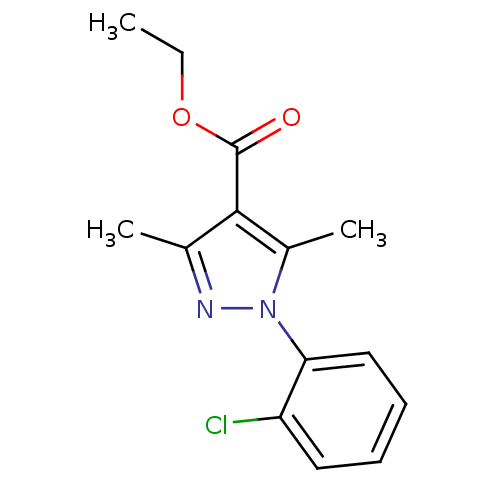
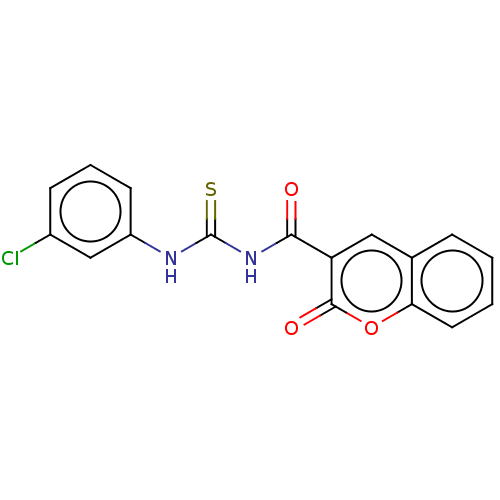
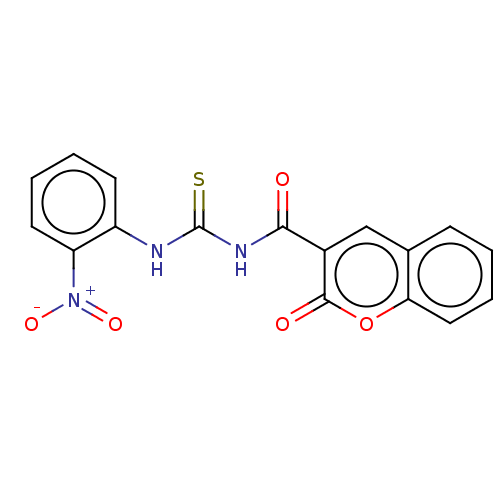
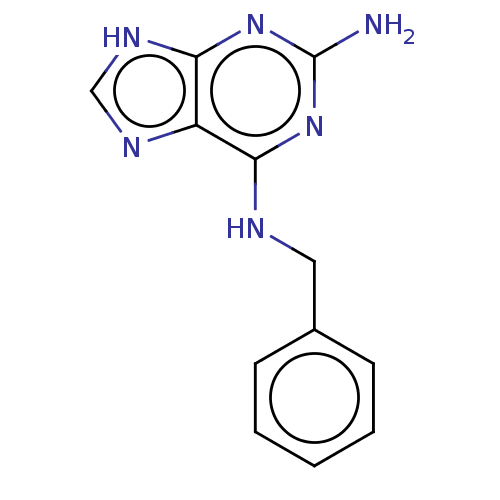
Affinity DataKi: 9nMAssay Description:Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr...More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr...More data for this Ligand-Target Pair
Affinity DataKi: 58nMAssay Description:Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft...More data for this Ligand-Target Pair
Affinity DataKi: 390nMAssay Description:Inhibition of Ostrinia furnacalis chitinase h catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by flu...More data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Inhibition of Serratia marcescens chitinase b catalytic domain overexpressed in Escherichia coli BL21(DE3) cells using 4MU-(GlcNAc)2 as substrate aft...More data for this Ligand-Target Pair
Affinity DataKi: 510nMAssay Description:Inhibition of mouse acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl...More data for this Ligand-Target Pair
Affinity DataKi: 1.12E+3nMAssay Description:Inhibition of human CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr...More data for this Ligand-Target Pair
Affinity DataKi: 1.38E+3nMAssay Description:Inhibition of mouse acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl...More data for this Ligand-Target Pair
Affinity DataKi: 1.94E+3nMAssay Description:Inhibition of mouse CHIT1 catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fluorescence based micr...More data for this Ligand-Target Pair
TargetAcidic mammalian chitinase(Homo sapiens (Human))
Dalian University of Technology
Curated by ChEMBL
Dalian University of Technology
Curated by ChEMBL
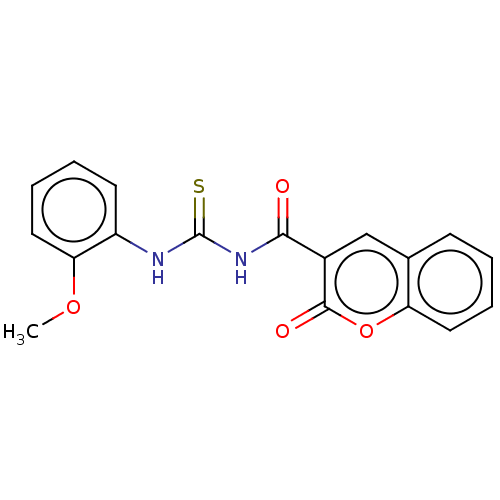
Affinity DataKi: 3.96E+3nMAssay Description:Inhibition of human acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl...More data for this Ligand-Target Pair
TargetAcidic mammalian chitinase(Homo sapiens (Human))
Dalian University of Technology
Curated by ChEMBL
Dalian University of Technology
Curated by ChEMBL
Affinity DataKi: 9.72E+3nMAssay Description:Inhibition of human acidic mammalian chitinase catalytic domain overexpressed in Pichia pastoris using 4MU-(GlcNAc)2 as substrate after 30 mins by fl...More data for this Ligand-Target Pair
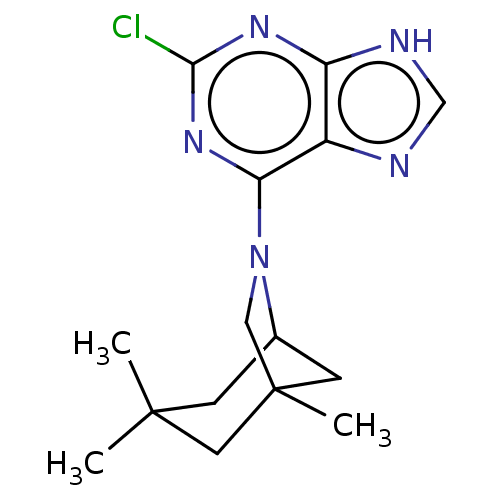
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 0.0210nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 0.0410nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 0.680nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 0.840nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q25B00Q1PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q25B00Q1PubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 2.20nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetIsoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) 35-489](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 10nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 11nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
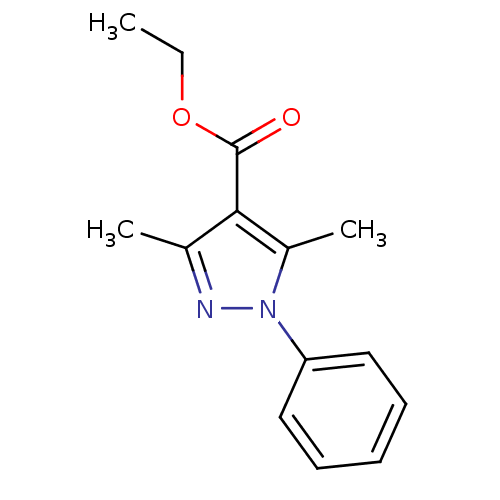
Affinity DataIC50: 13nMpH: 7.0 T: 2°CAssay Description:The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 19nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 21nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 25nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
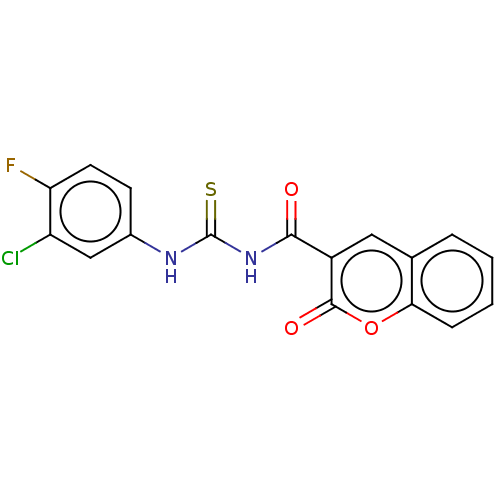
Affinity DataIC50: 30nMAssay Description:Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 33nMpH: 7.5 T: 2°CAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 56nMpH: 7.5 T: 2°CAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
In DepthDetails
Article
BindingDB Entry DOI: 10.7270/Q21N7ZCNPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)
BindingDB Entry DOI: 10.7270/Q21N7ZCNPubMedDrugBank
MMDB
PDB
 3D Structure (crystal)
3D Structure (crystal)Affinity DataIC50: 60nMAssay Description:Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMpH: 7.5 T: 2°CAssay Description:Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) according to the manufacturer instructions.More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 160nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMpH: 7.0 T: 2°CAssay Description:The in vitro kinase activities of wild type or mutants were determined by measuring phosphorylation of biotinylated-MEK protein using Perkin-Elmer s ...More data for this Ligand-Target Pair
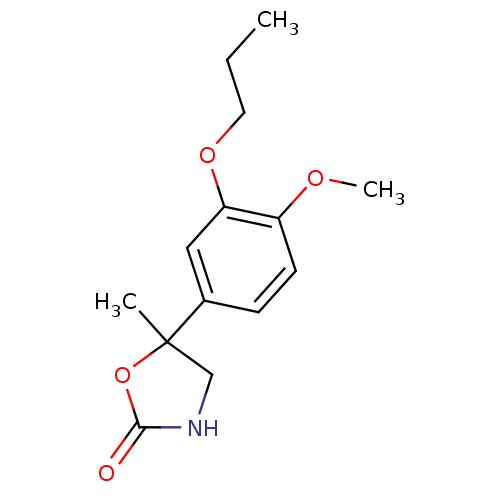
TargetOxidized purine nucleoside triphosphate hydrolase(Homo sapiens (Human))
RIKEN Center for Life Science Technologies
RIKEN Center for Life Science Technologies
Affinity DataIC50: 210nMpH: 7.5Assay Description:Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r...More data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ...More data for this Ligand-Target Pair
TargetOxidized purine nucleoside triphosphate hydrolase(Homo sapiens (Human))
RIKEN Center for Life Science Technologies
RIKEN Center for Life Science Technologies
Affinity DataIC50: 230nMpH: 7.5Assay Description:Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r...More data for this Ligand-Target Pair
TargetIsoform 4 of Dual 3',5'-cyclic-AMP and -GMP phosphodiesterase 11A (PDE11A1) 35-489](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 240nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 270nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 300nMpH: 7.5 T: 2°CAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 310nMpH: 7.5 T: 2°CAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetOxidized purine nucleoside triphosphate hydrolase(Homo sapiens (Human))
RIKEN Center for Life Science Technologies
RIKEN Center for Life Science Technologies
Affinity DataIC50: 350nMpH: 7.5Assay Description:Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 350nMpH: 7.5 T: 2°CAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D [372-376,381-715,S375G,I381V,S383G,G384S,V385H,K386M)(Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 390nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetOxidized purine nucleoside triphosphate hydrolase(Homo sapiens (Human))
RIKEN Center for Life Science Technologies
RIKEN Center for Life Science Technologies
Affinity DataIC50: 420nMpH: 7.5Assay Description:Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 420nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B [316-320,321-700,S319G,N320S,N321H,T322M](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 570nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
TargetOxidized purine nucleoside triphosphate hydrolase(Homo sapiens (Human))
RIKEN Center for Life Science Technologies
RIKEN Center for Life Science Technologies
Affinity DataIC50: 630nMpH: 7.5Assay Description:Enzymatic reaction was initiated by adding either 8-oxo-dGTP (13.2 μm; TriLink BioTechnologies) or 2-OH-dATP (8.3 μm; Jena Bioscience) to r...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A [226-593](Homo sapiens (Human))
Plexxikon
Plexxikon
Affinity DataIC50: 680nMAssay Description:Phosphodiesterase activities were assayed in the presence of inhibitor compounds. Measurement takes advantage of the selective binding of 5-AMP or 5-...More data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Newly synthesized coumarin thioureas were tested against electric eel AChE and horse serum BChE. The cholinesterase inhibitory activity was measured ...More data for this Ligand-Target Pair