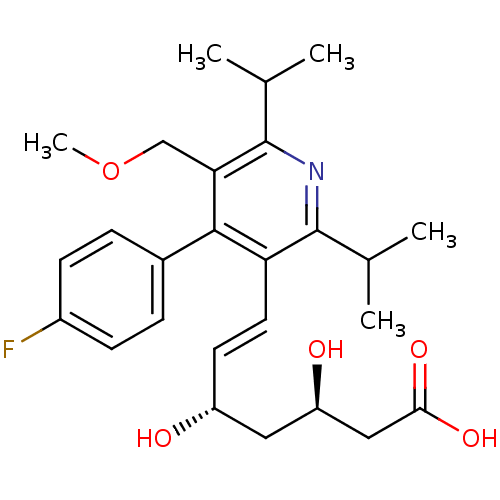
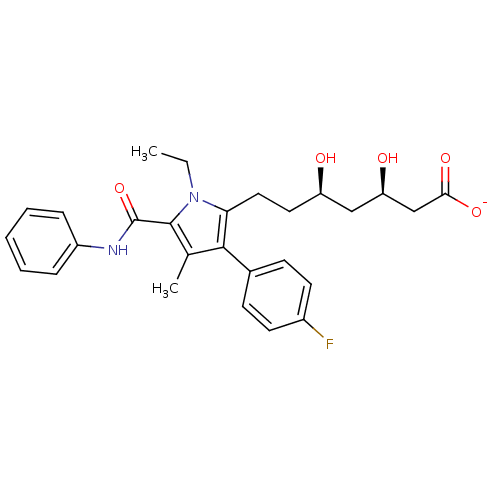
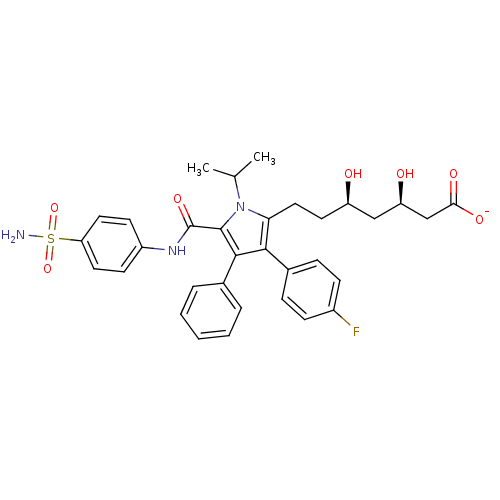
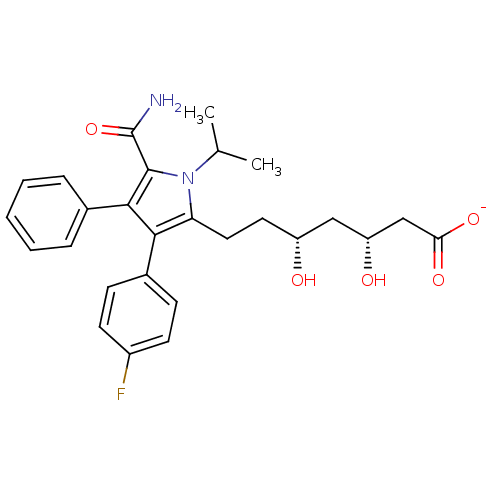
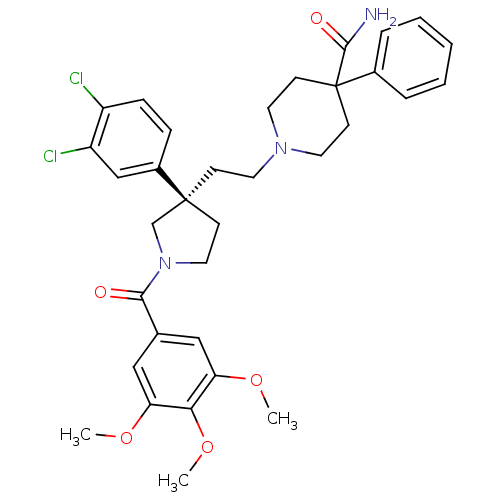
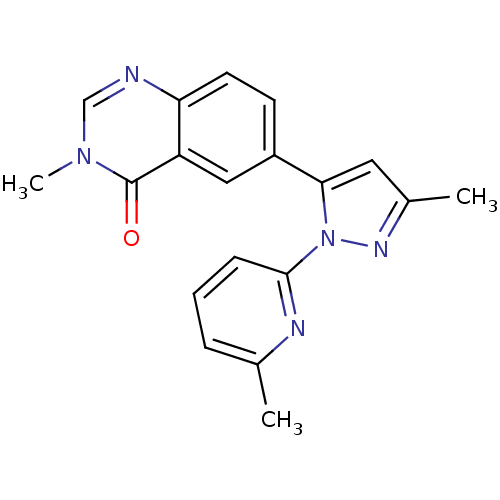
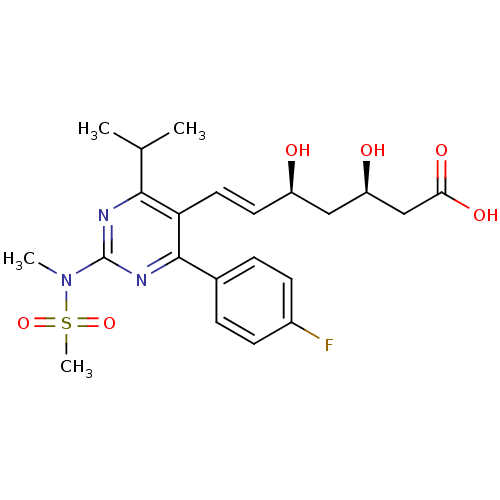
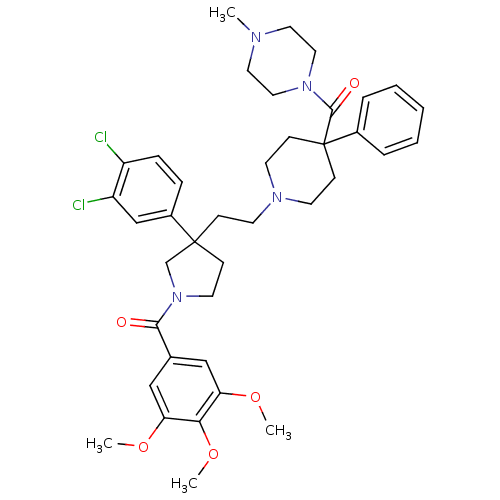
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Compound was evaluated for the antagonistic activity against NK1 receptorMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:Antagonism of the guinea pig tachykinin NK1 receptorMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair
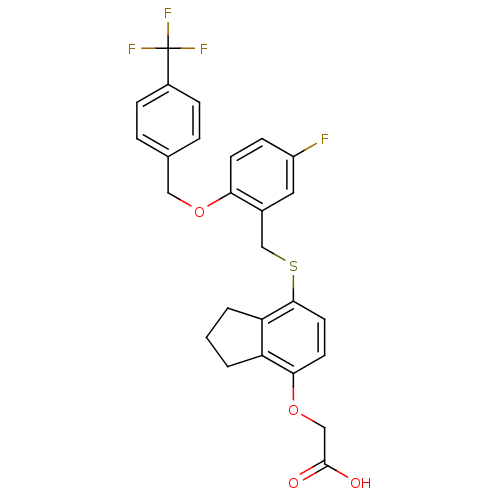
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPA assayMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA.More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA.More data for this Ligand-Target Pair
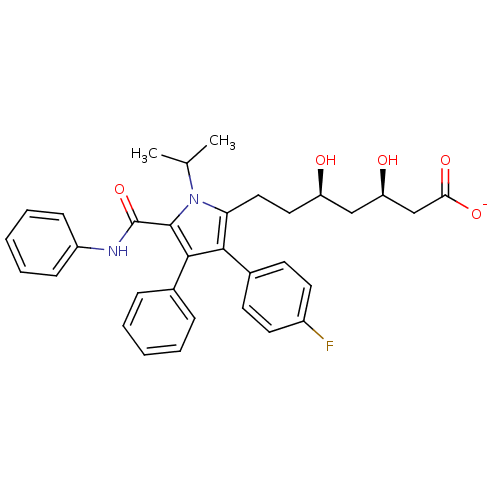
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SPMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SPMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPAMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair
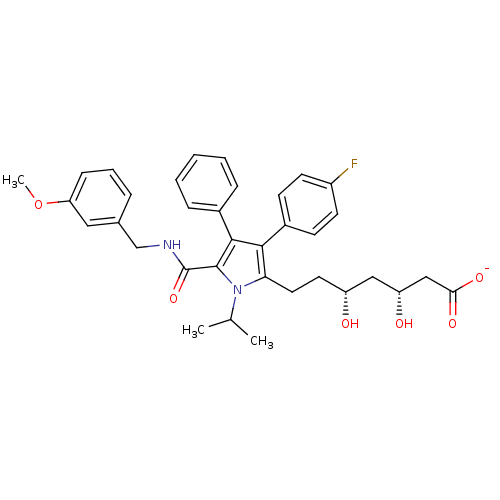
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Compound was evaluated for the antagonistic activity against NK1 receptorMore data for this Ligand-Target Pair
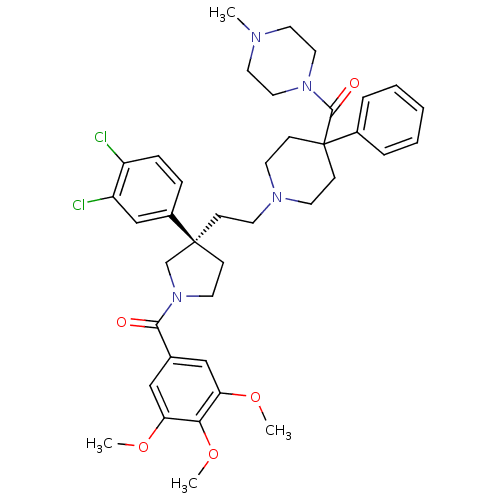
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA.More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Binding affinity for NK1 receptor in guinea pig lung was determined by using [125 I]-Bolton Hunter labeled SPMore data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Homo sapiens (Human))
Pfizer Global Research & Development
Curated by ChEMBL
Pfizer Global Research & Development
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of HMG-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Binding affinity for NK2 receptor in HSKR-1 cells was determined by using [125 I ]-Iodohistidyl NKA.More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Displacement of [3H]2-(4-(3-(4-acetyl-3-hydroxy-2 propyl-phenoxy)propoxy)phenoxy)acetic acid from human PPARdelta after 30 mins by SPAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMpH: 7.2 T: 2°CAssay Description:Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme source...More data for this Ligand-Target Pair




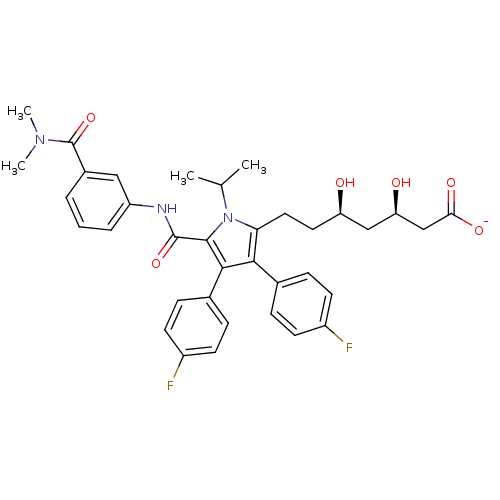




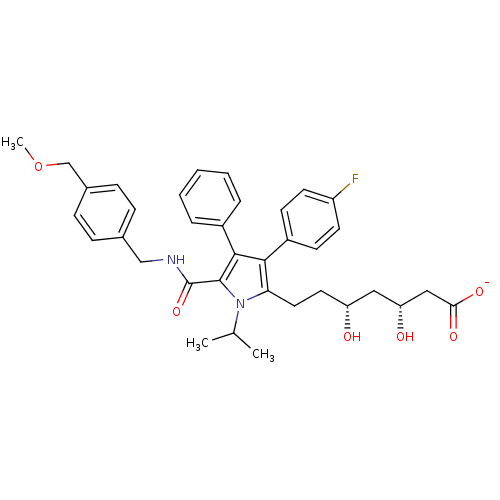
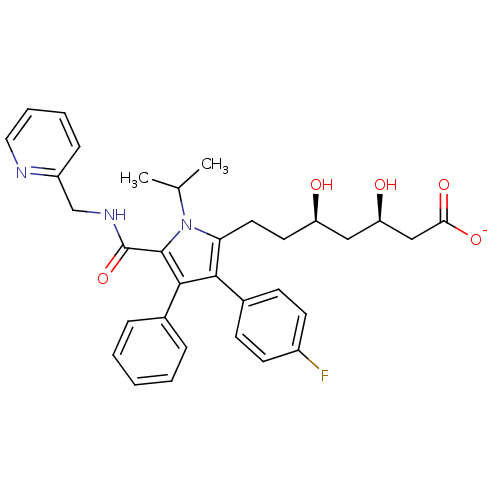
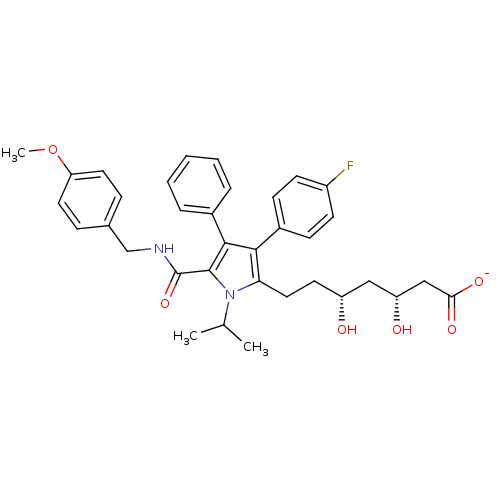

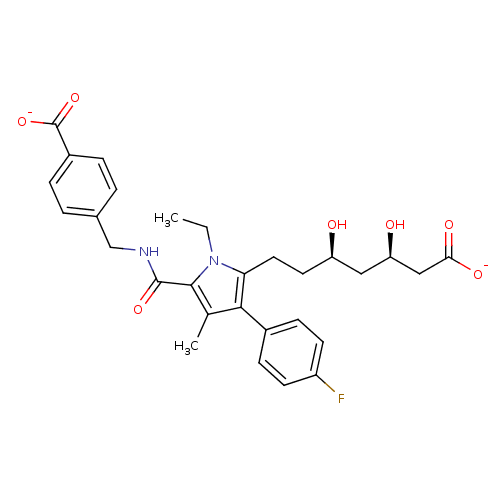
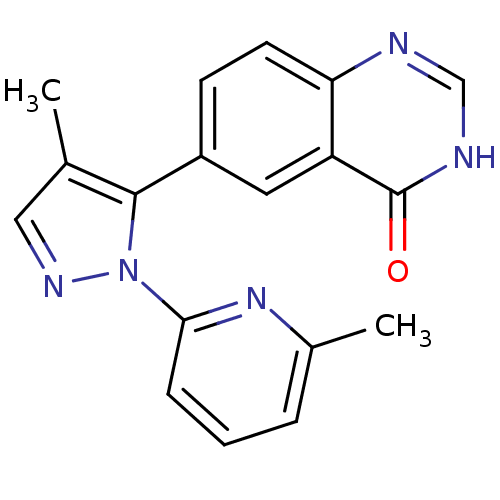
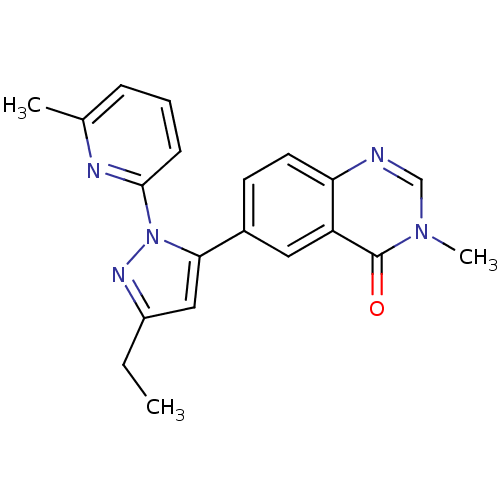
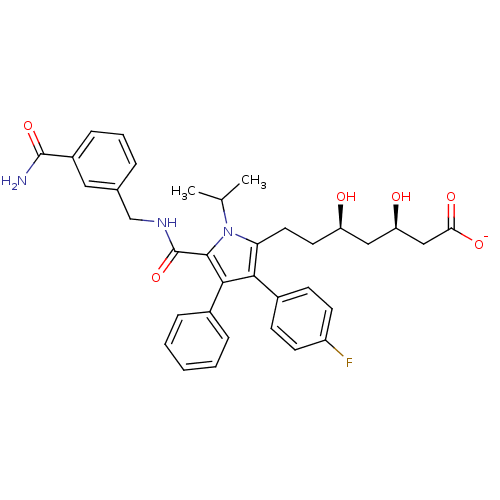

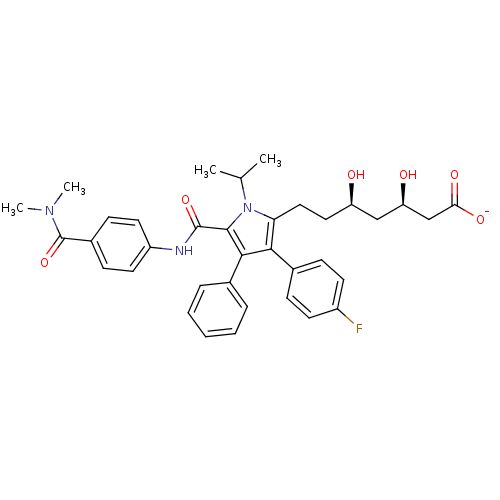
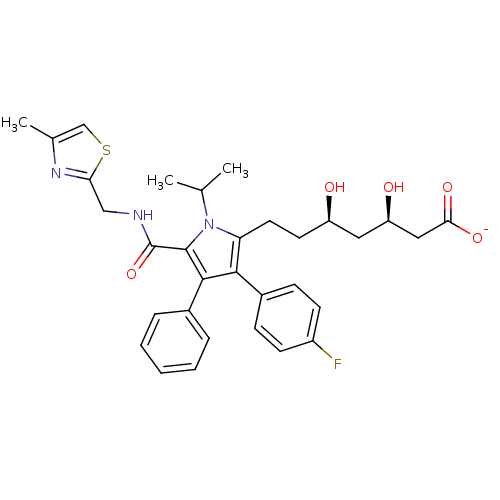
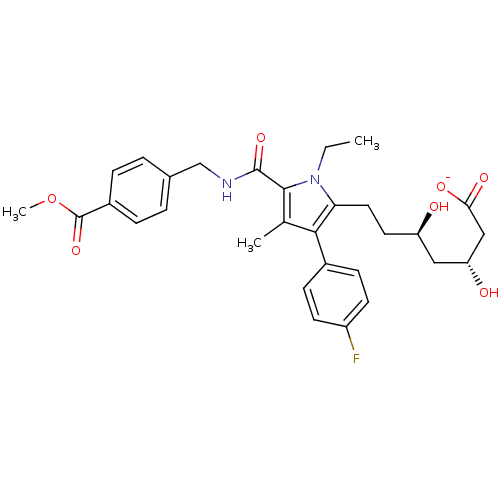
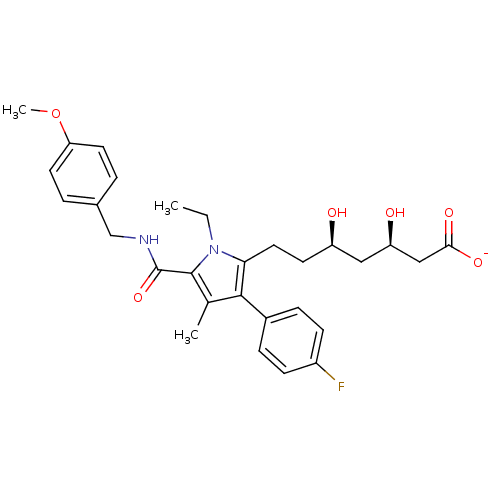




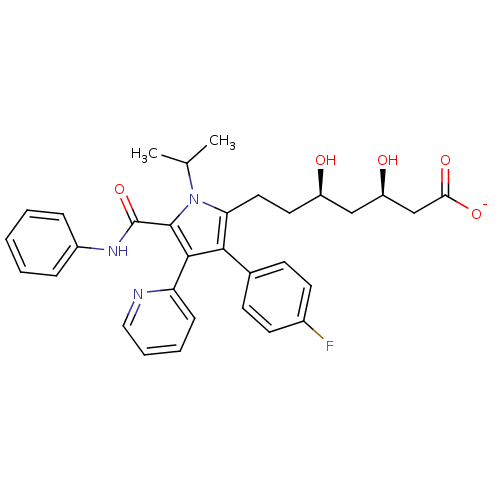

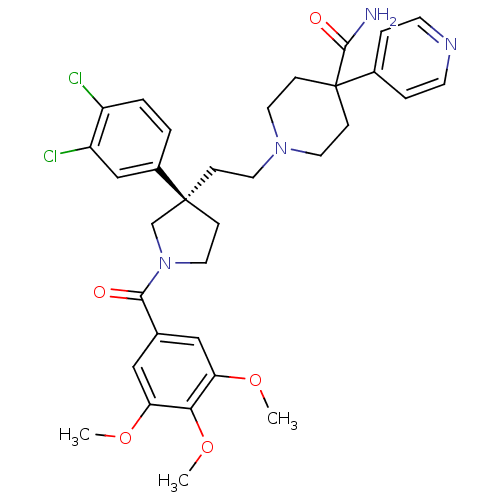

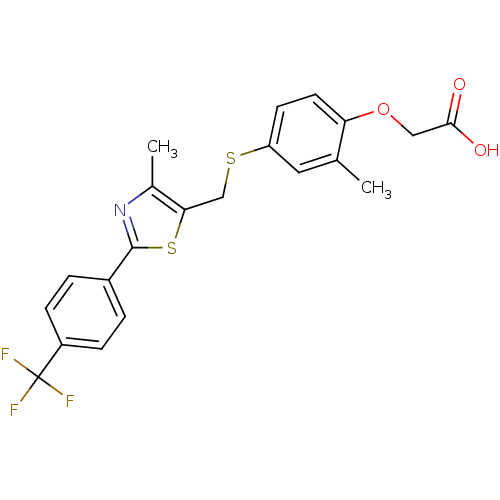
 3D Structure (crystal)
3D Structure (crystal)