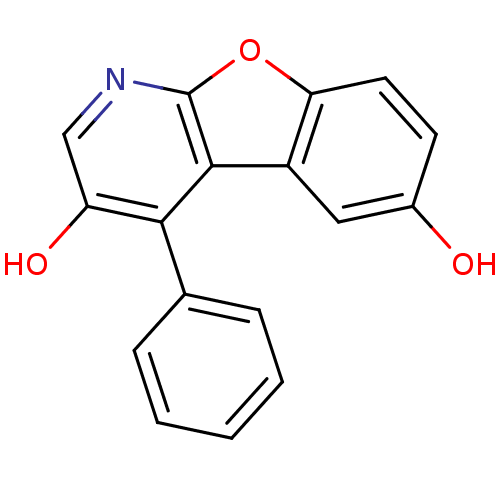
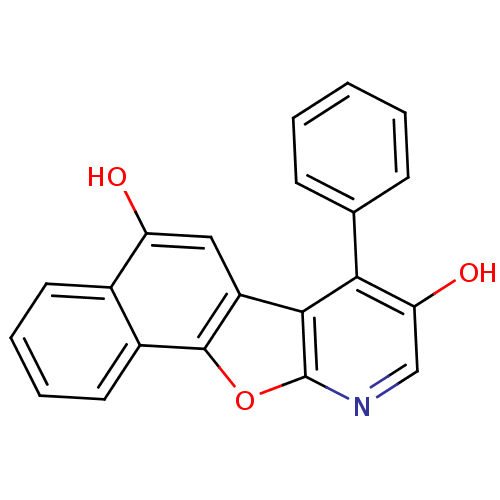
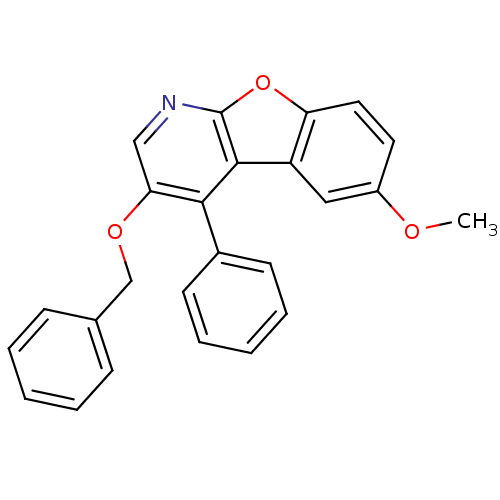
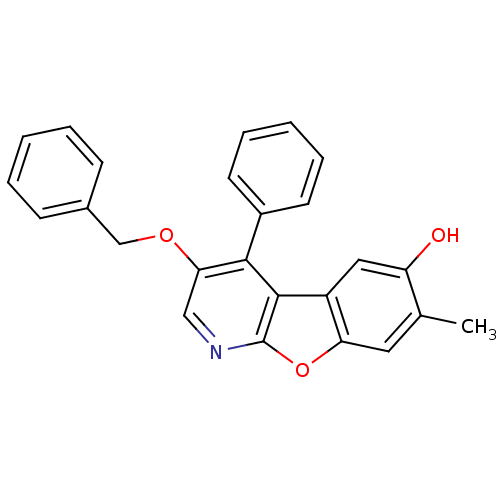
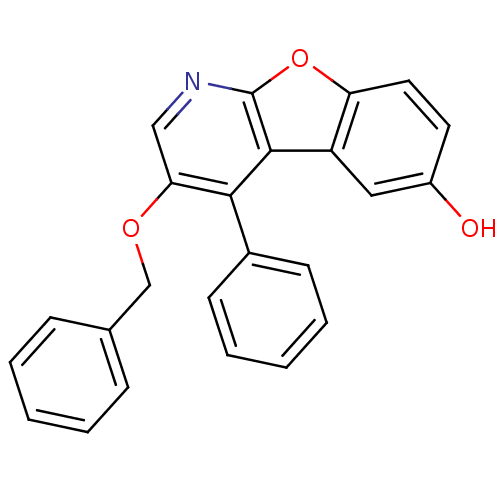
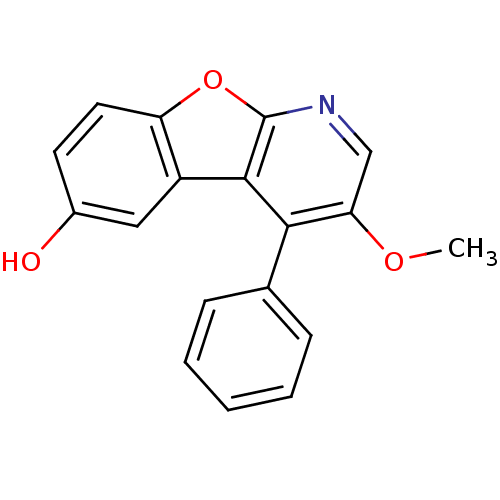
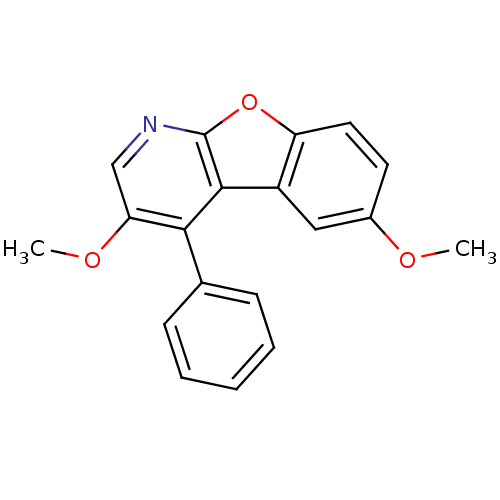
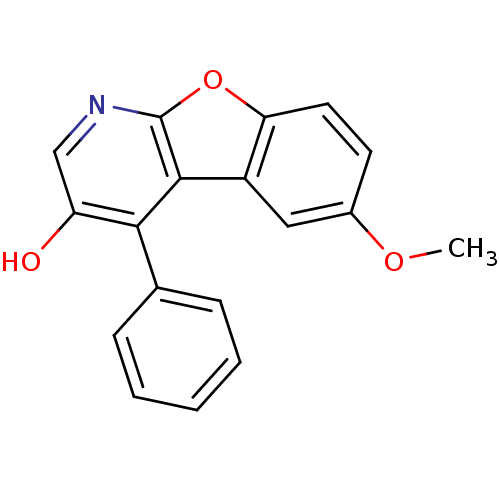
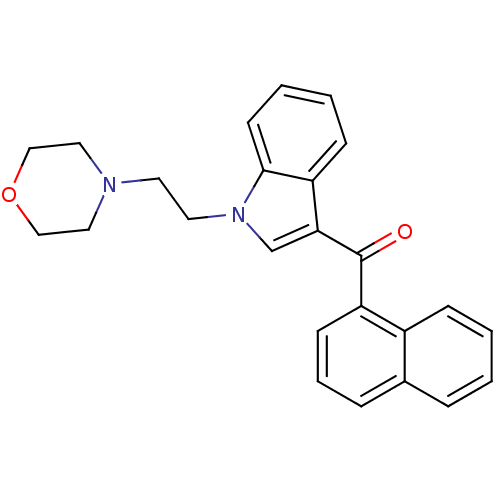
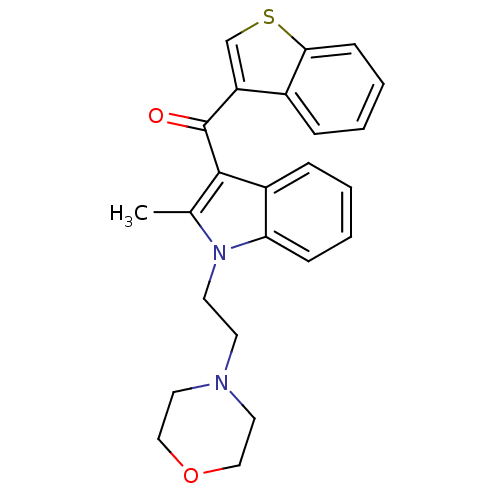
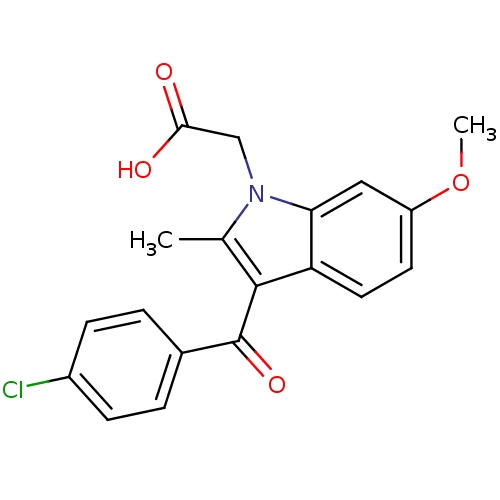
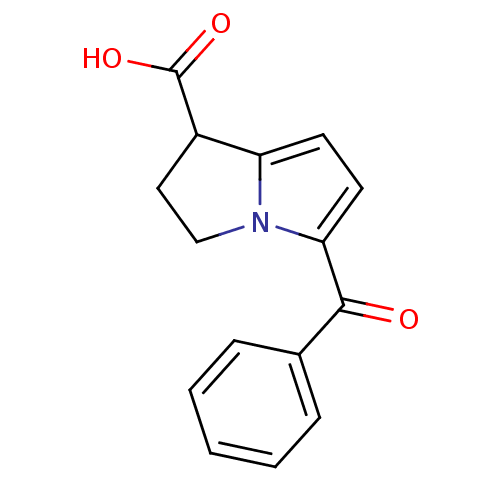
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of GSK3betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 5.30E+3nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 5.80E+3nMAssay Description:Inhibition of GSK3betaMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 5.80E+3nMAssay Description:Inhibition of GSK3betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 6.40E+3nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 1.48E+4nMAssay Description:Inhibition of GSK3betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.40E+4nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.53E+4nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.61E+4nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 1.47E+5nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.17E+5nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
TargetCyclin-dependent kinase 6/G1/S-specific cyclin-D1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 7.73E+5nMAssay Description:Inhibition of CDK6/Cyclin D1More data for this Ligand-Target Pair
TargetProtein kinase C gamma type(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of PKCgammaMore data for this Ligand-Target Pair
TargetProtein kinase C epsilon type(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of PKCepsilonMore data for this Ligand-Target Pair
TargetProtein kinase C iota type(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of PKCiotaMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of VEGFR2More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of ERBB2More data for this Ligand-Target Pair
TargetAngiopoietin-1 receptor(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of TIE2More data for this Ligand-Target Pair
TargetWee1-like protein kinase(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of WEE1More data for this Ligand-Target Pair
TargetCasein kinase I isoform alpha(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of Ck1alpha1More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of GSK3betaMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of GSK3betaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK2/Cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK5/p25More data for this Ligand-Target Pair
TargetProtein kinase C alpha type(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of PKCalphaMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3(Homo sapiens (Human))
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: >1.00E+6nMAssay Description:Inhibition of CDK1/Cyclin BMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomesMore data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Concentration required to inhibit 50% activity of prostaglandin synthetase was determined in vitro in mouse brain microsomesMore data for this Ligand-Target Pair