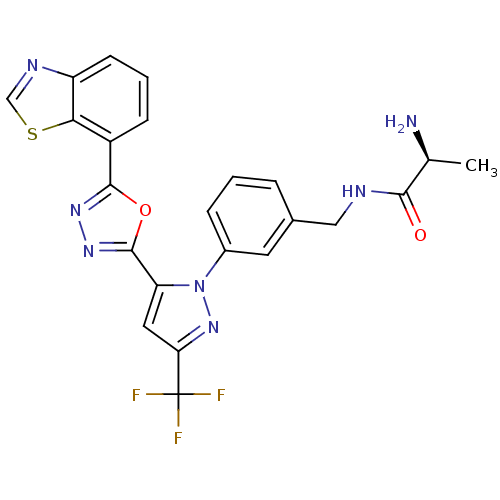
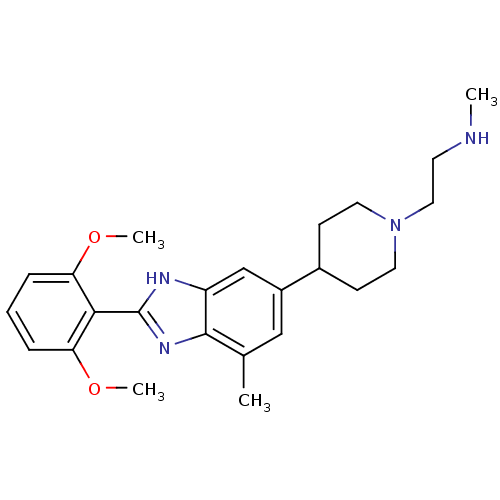
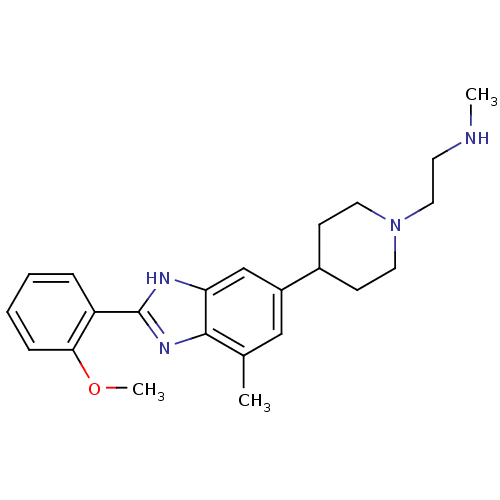
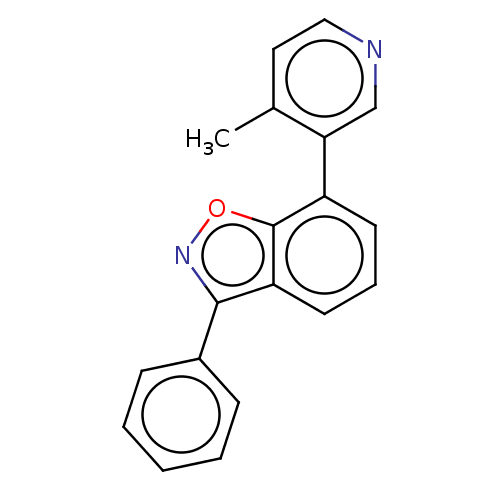
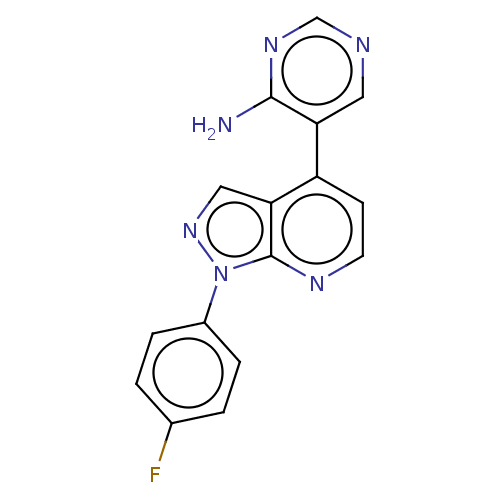
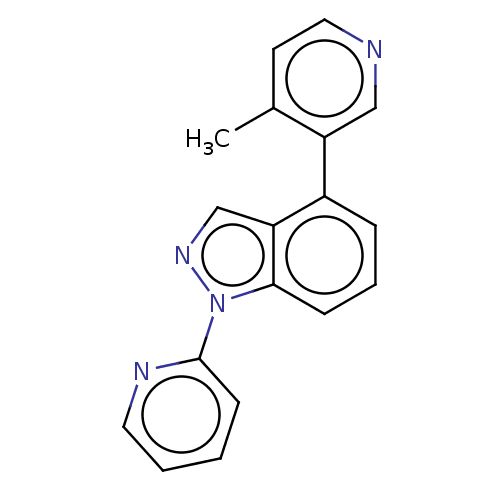
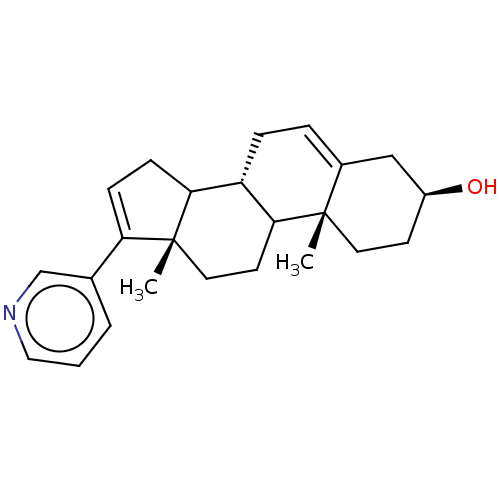
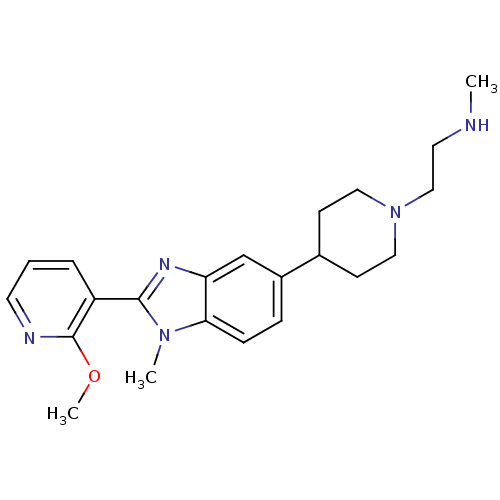
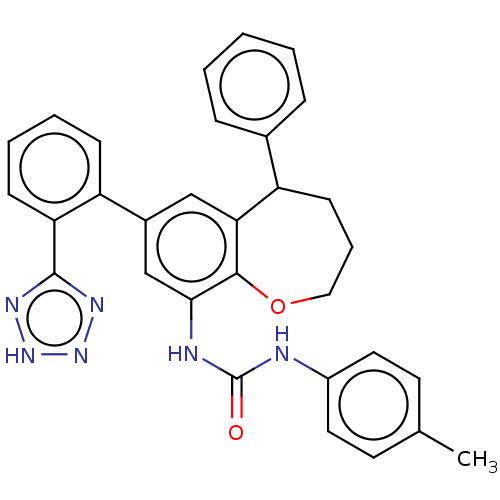
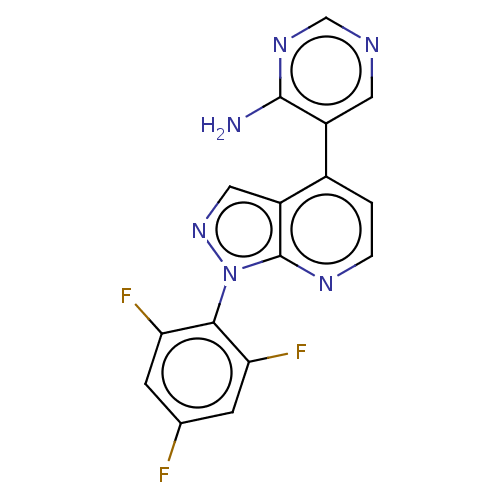
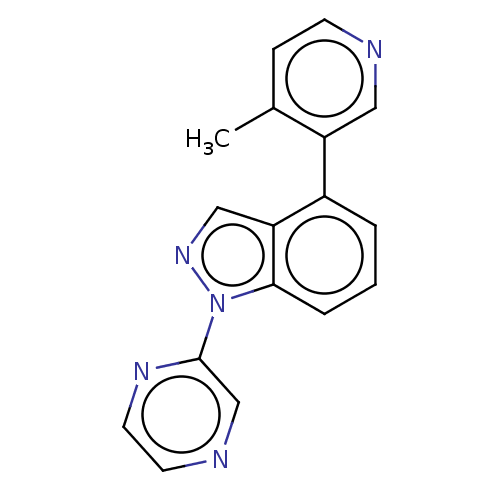
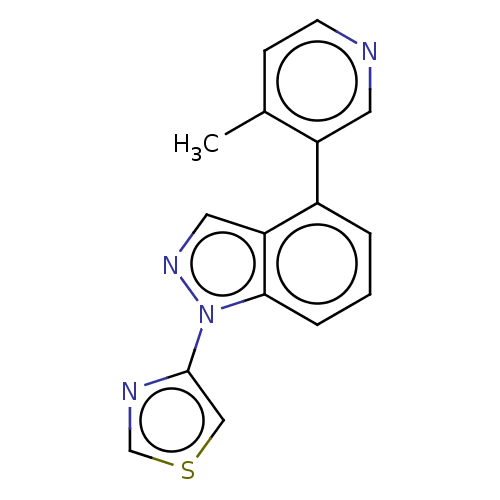
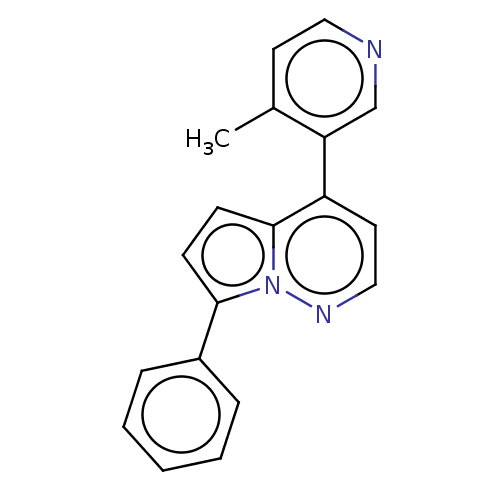
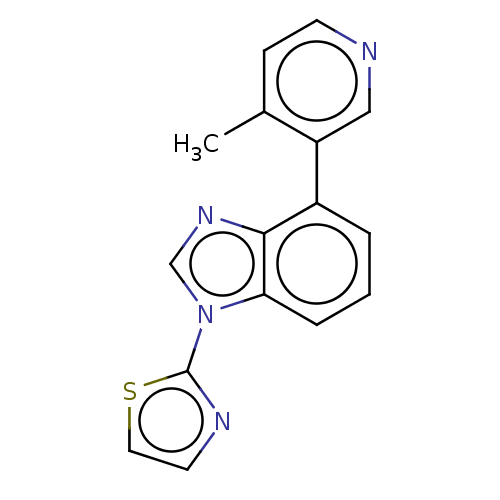
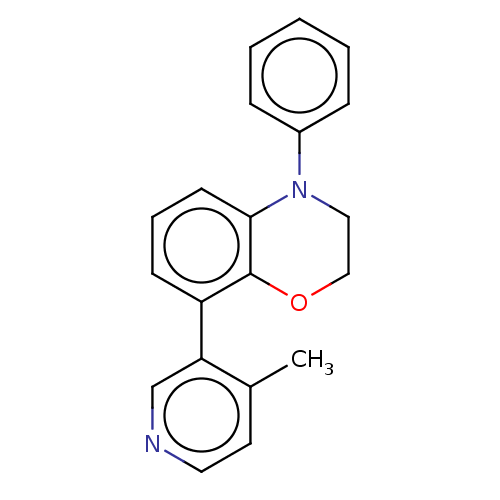
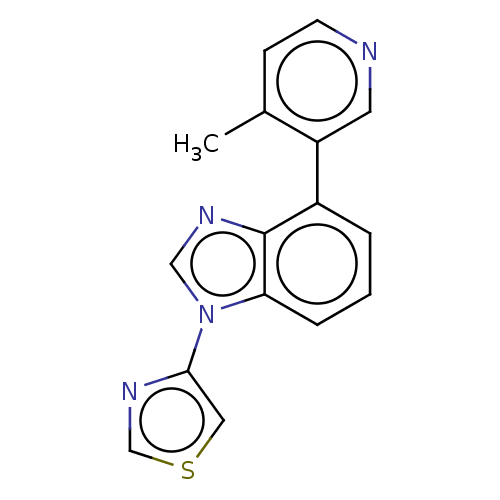
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
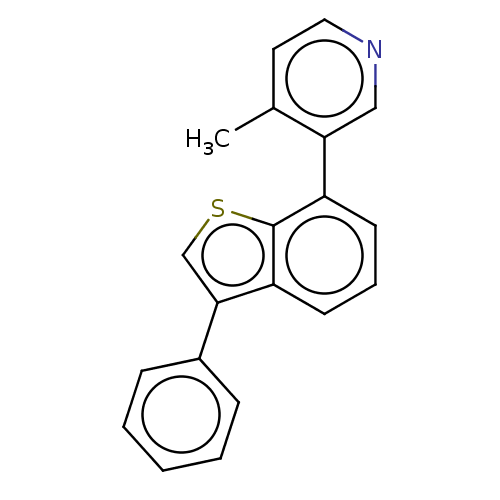
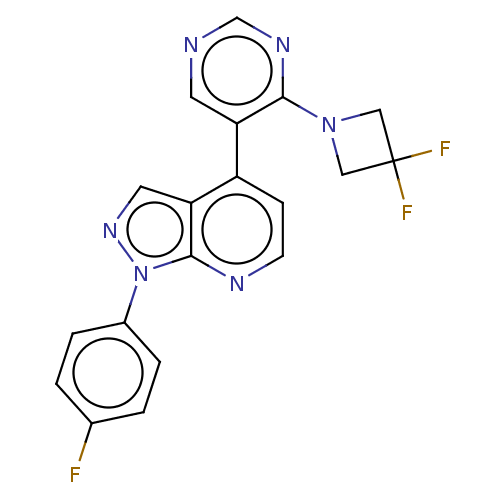
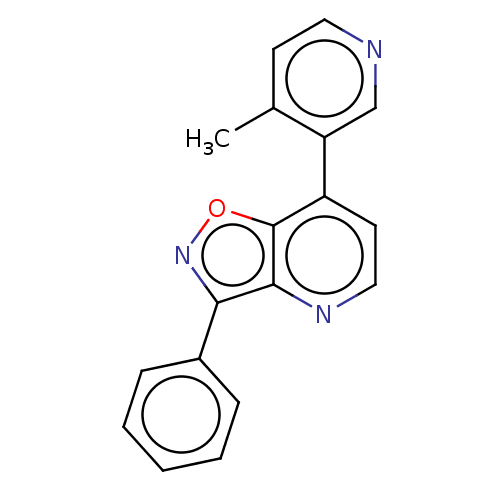
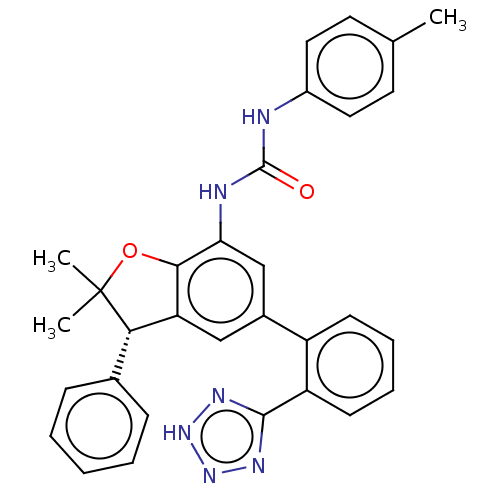
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
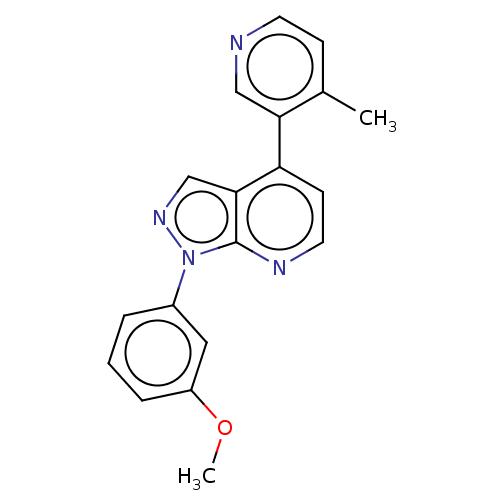
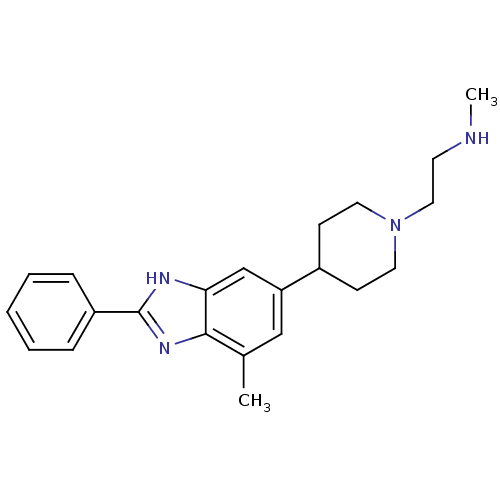
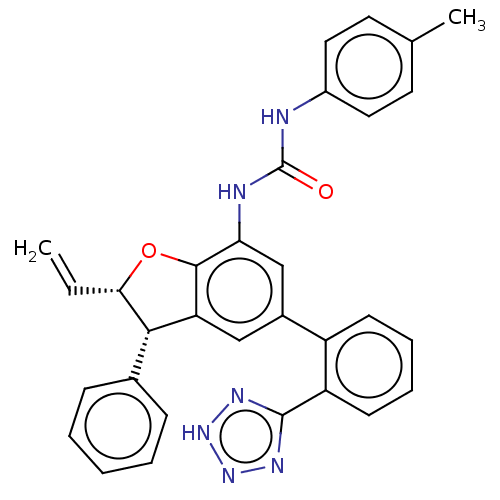
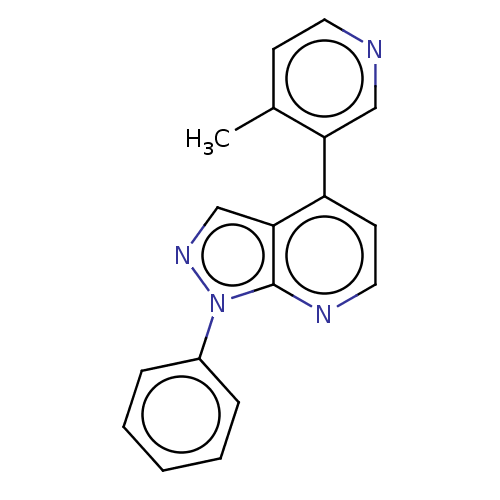
Affinity DataIC50: 0.0700nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
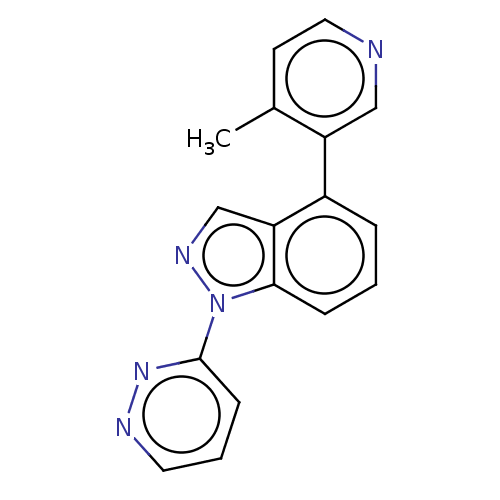
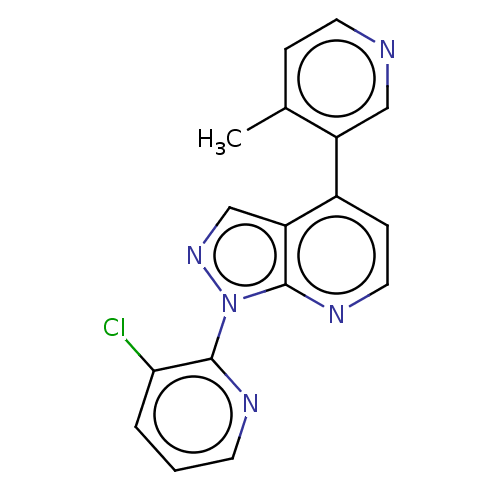
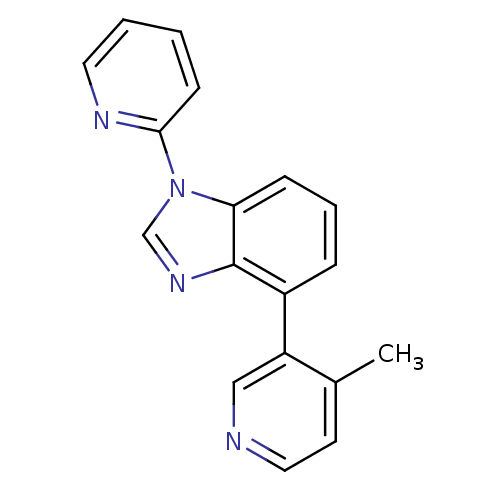
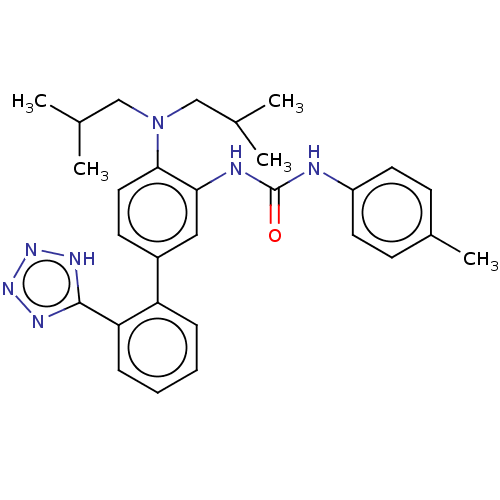
Affinity DataIC50: 0.120nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
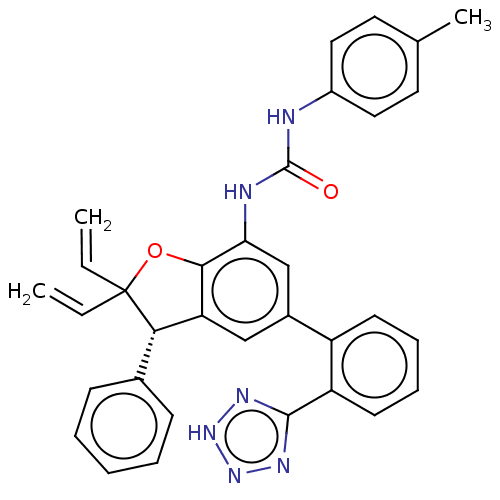
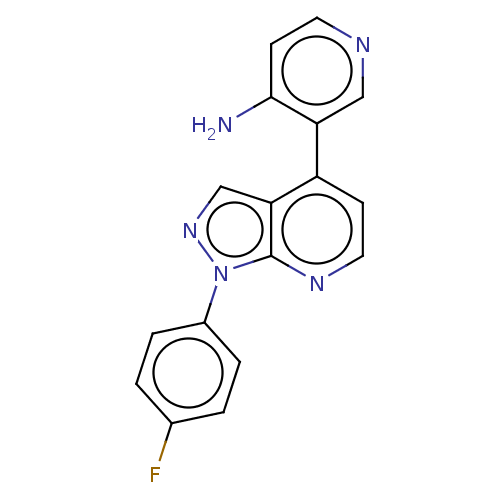
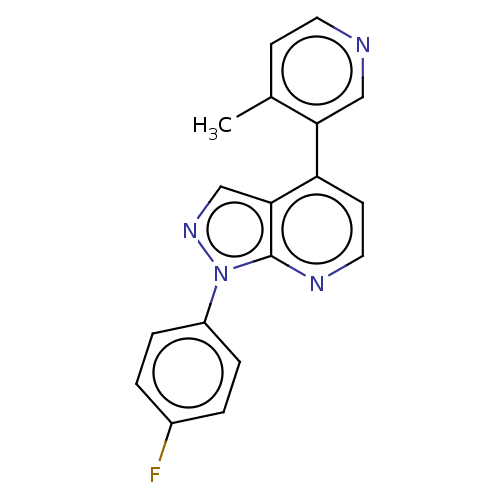
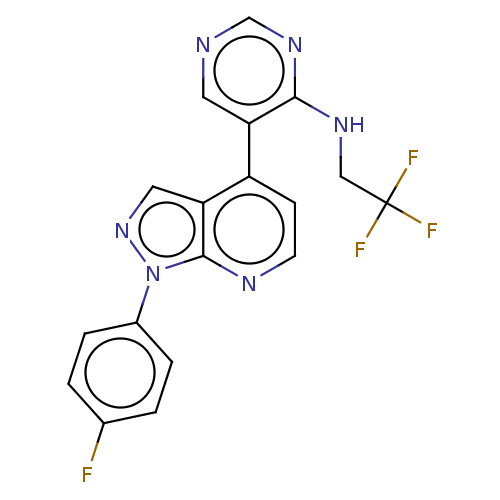
Affinity DataIC50: 0.260nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.410nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.840nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against cell free dihydrofolate reductase (DHFR) from ratMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufuMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 2nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey adrenal microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity a...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 3nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 3nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 4nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brainMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 4nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brainMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 4nMAssay Description:Inhibition of CYP17A1 in cynomolgus monkey using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity assay in presence of...More data for this Ligand-Target Pair
TargetHistone-arginine methyltransferase CARM1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human CARM1 assessed as inhibition of histone3 methylationMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 5nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to A9 L cells transfected with muscarinic M1 receptor of rat brainMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 5nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to muscarinic M2 receptor of rat heartMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 5nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brainMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 5nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 5nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 6nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to muscarinic M2 receptor of rat heartMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 6nMAssay Description:Inhibitory activity against cell free dihydrofolate redutase (DHFR) from Mycobacterium lufuMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 7nMAssay Description:Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of radioligand [3H]GR-65630.More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
Affinity DataIC50: 8nMAssay Description:Binding affinity by the displacement of [3H]NMS binding to muscarinic M1 receptor of rat brainMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 8nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity against Melatonin receptor using ovine pars tuberalis membranes of the pituitary.More data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 8nMAssay Description:Inhibitory activity against dihydropteroic acid synthase (SYN) from Plasmodium bergheiMore data for this Ligand-Target Pair
In DepthDetails
Affinity DataIC50: 8nMAssay Description:Inhibition of human CYP17A1 expressed in HEK293 cell microsomes using [3H]-pregnenolone as substrate incubated for 45 mins by scintillation proximity...More data for this Ligand-Target Pair