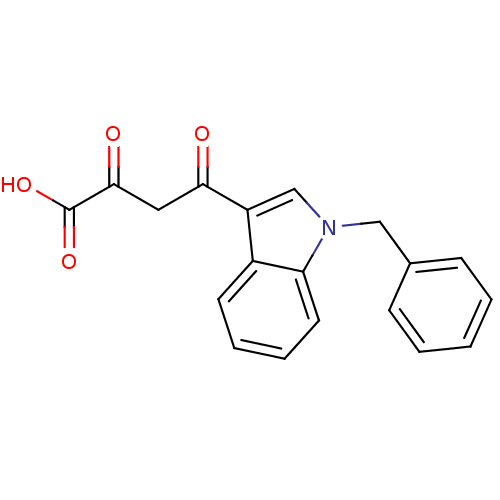
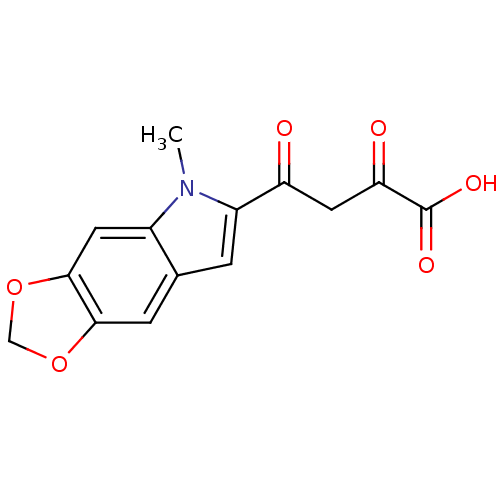
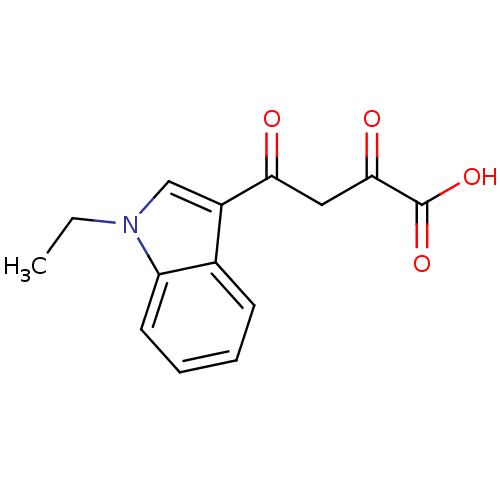
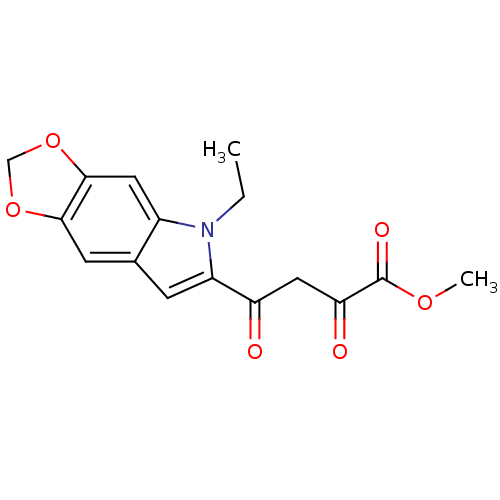
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated strand transfer reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 8.50E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 9.30E+4nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibitory concentration against HIV-1 integrase mediated 3' processing reactionMore data for this Ligand-Target Pair