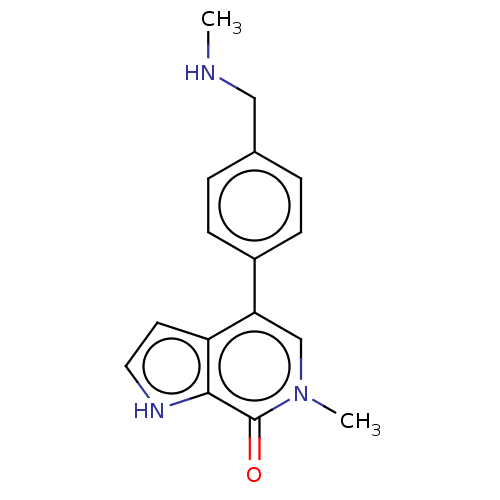
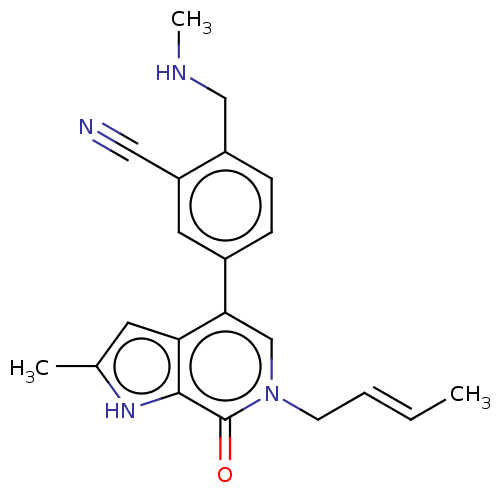
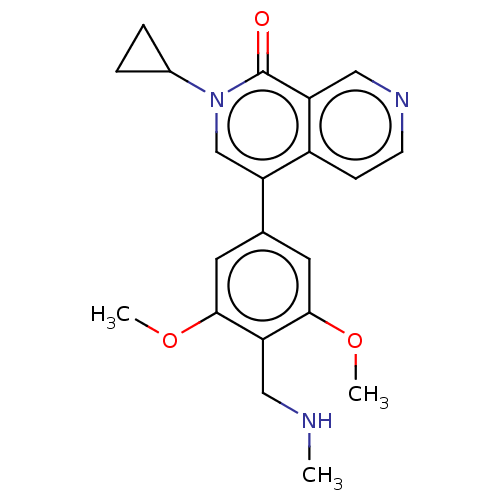
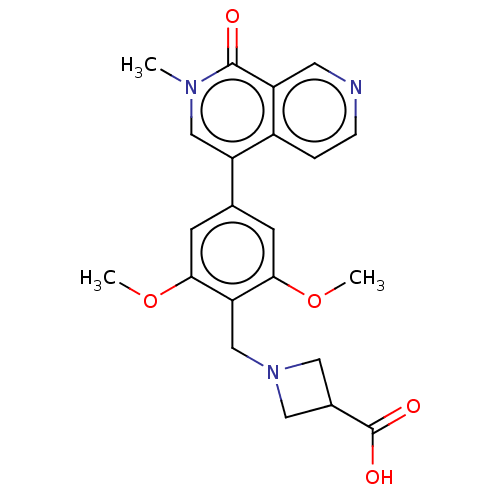
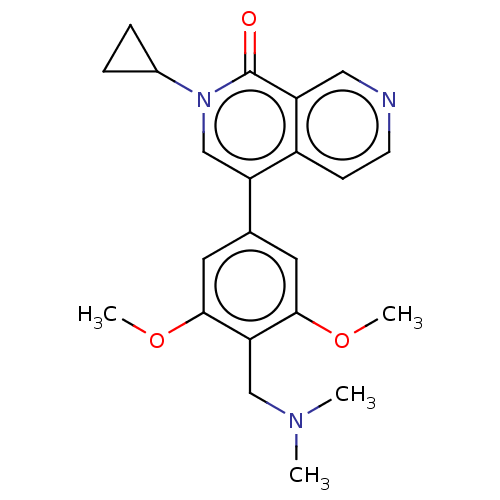
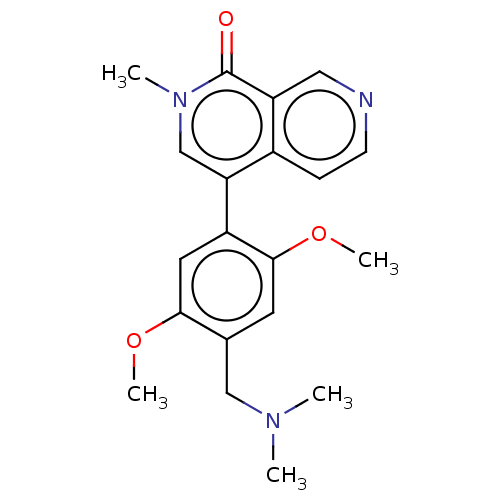
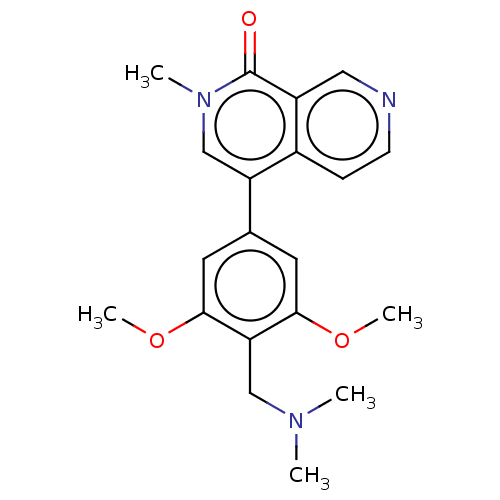
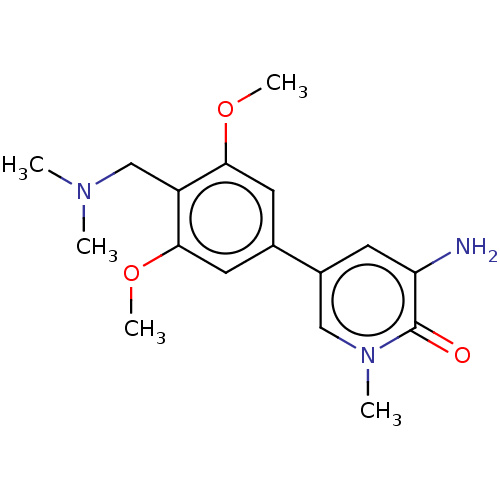
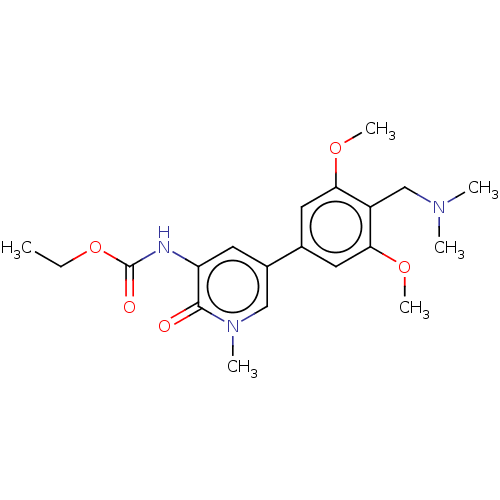
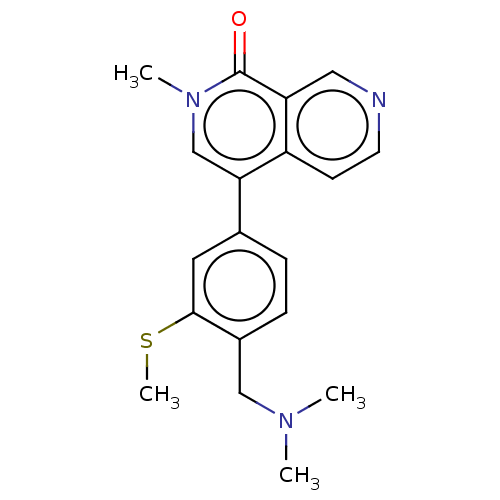
Affinity DataIC50: 0.0352nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 0.177nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 0.265nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
Affinity DataIC50: 11nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Inhibition of human recombinant HDAC6 using ZMAL (Z-(Ac)Lys-AMC fluorogenic substrate incubated for 90 mins by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho...More data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails



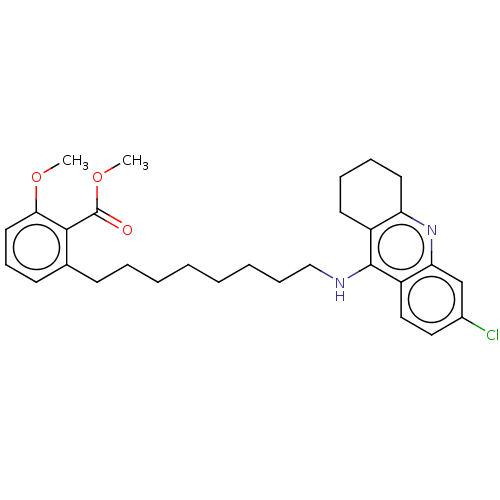
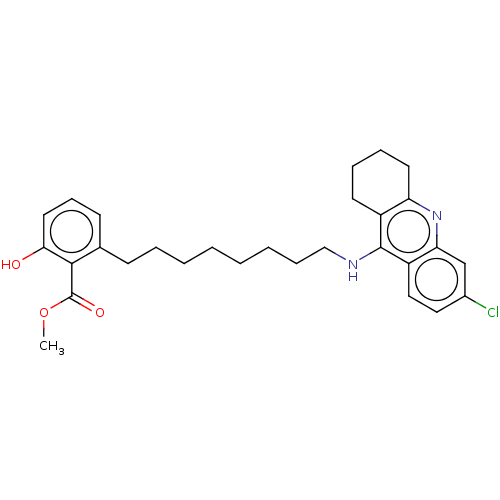
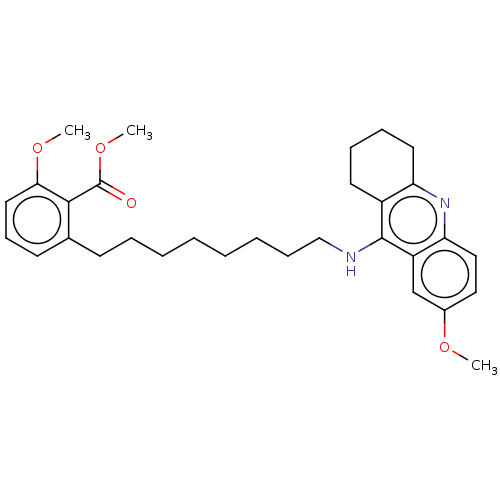

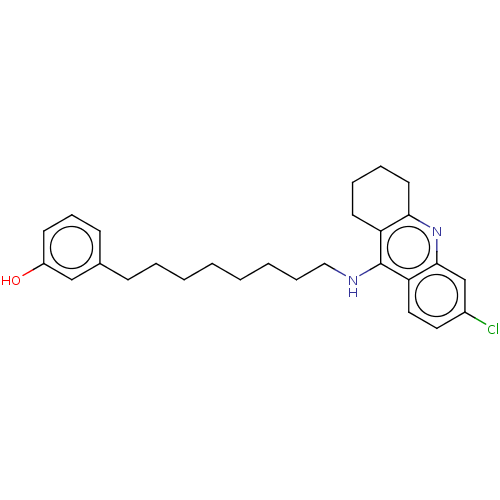
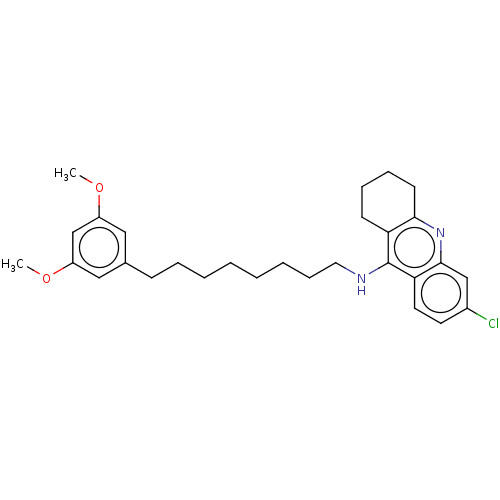


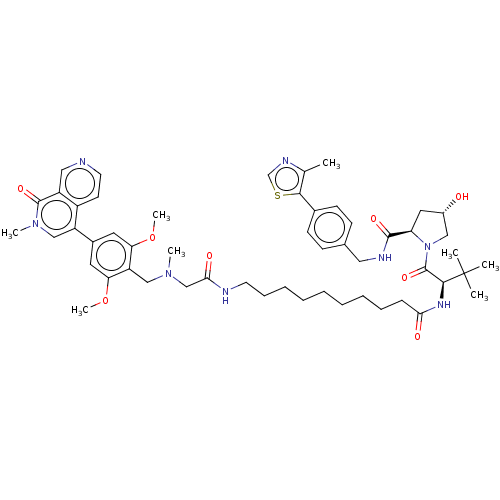
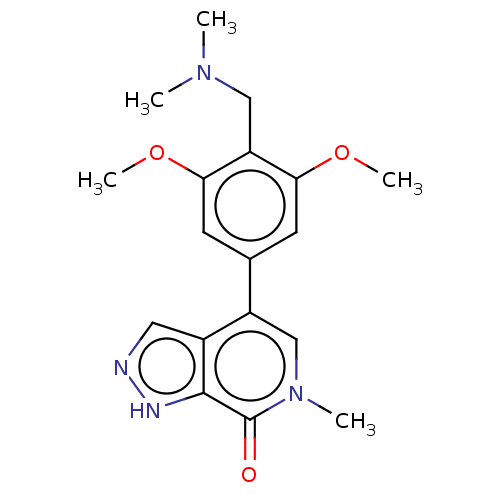

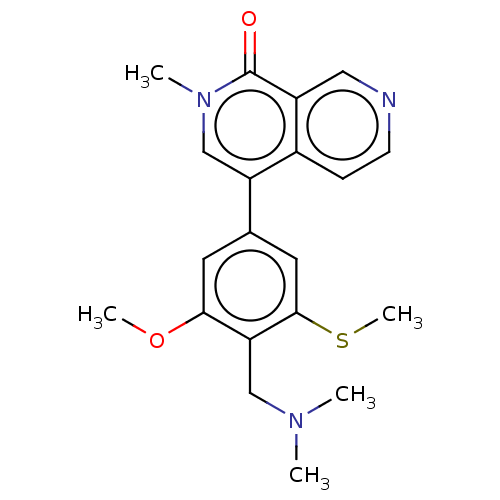

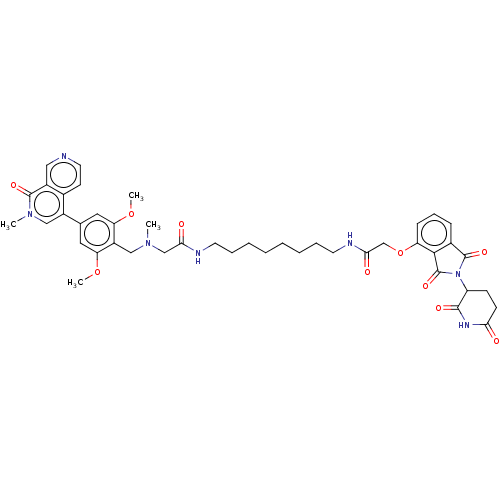

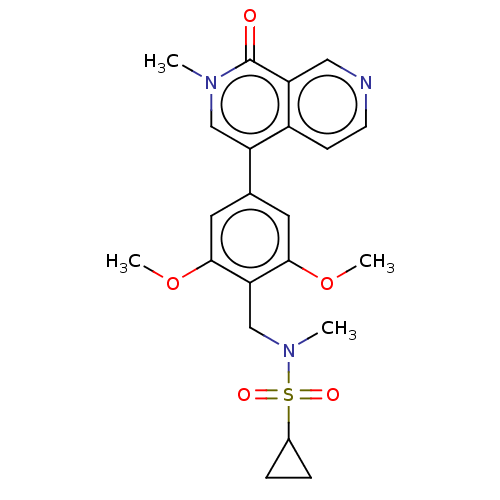


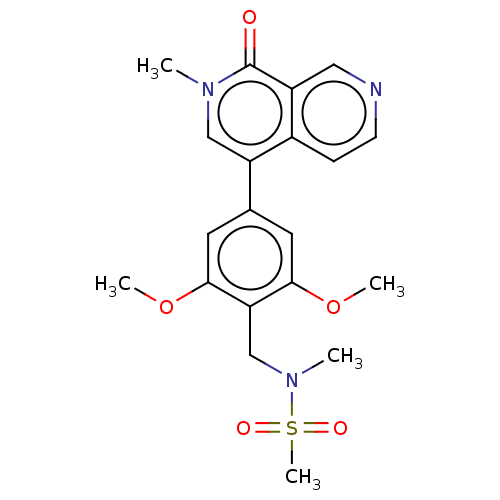
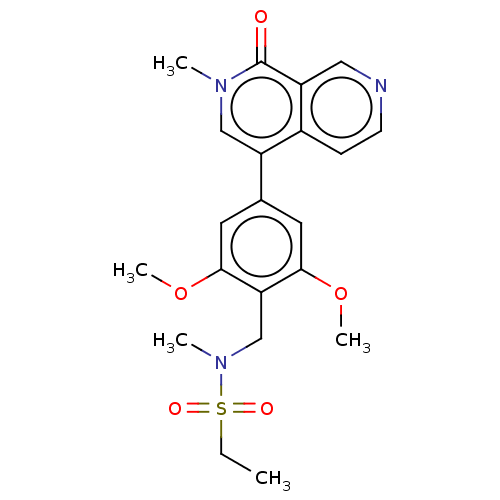
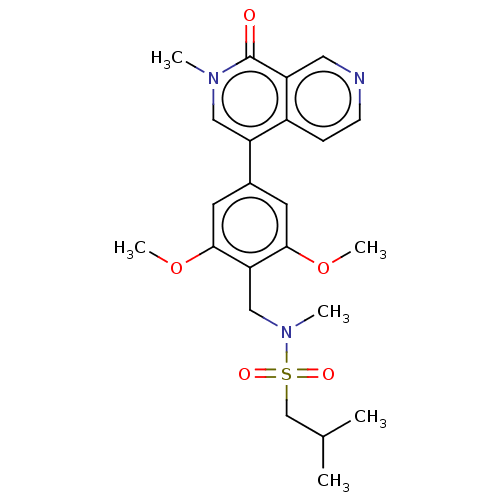
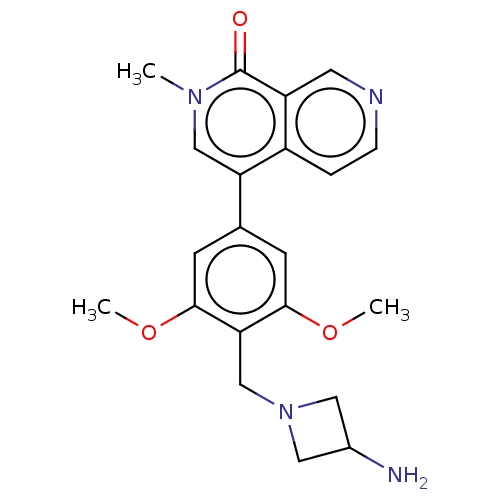
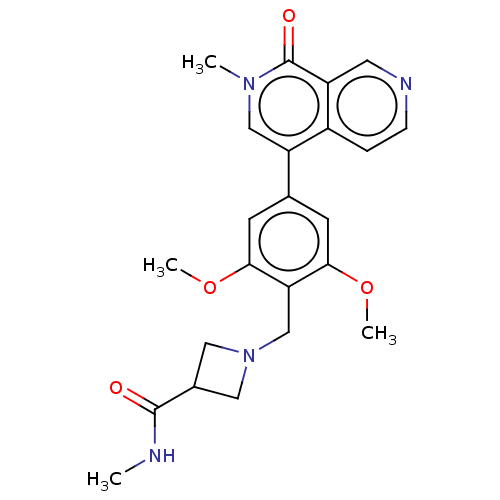
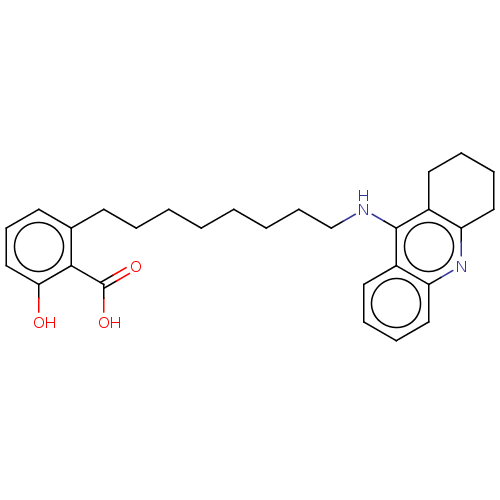
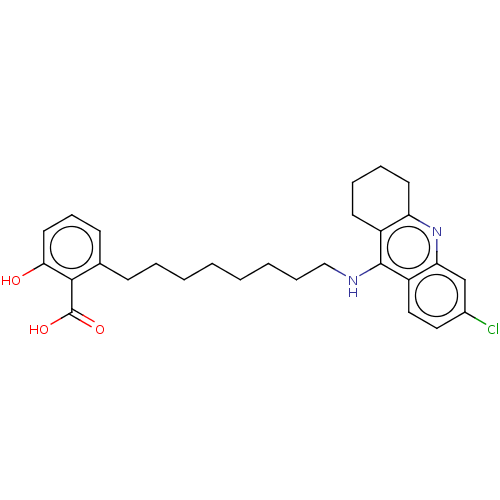

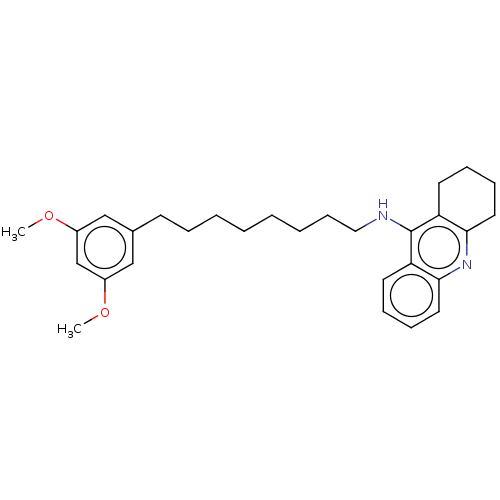


 3D Structure (crystal)
3D Structure (crystal)