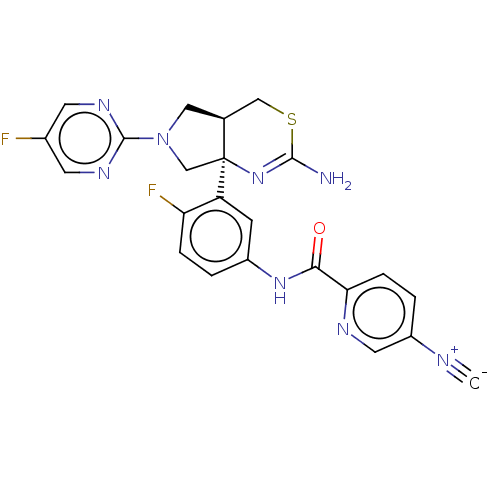
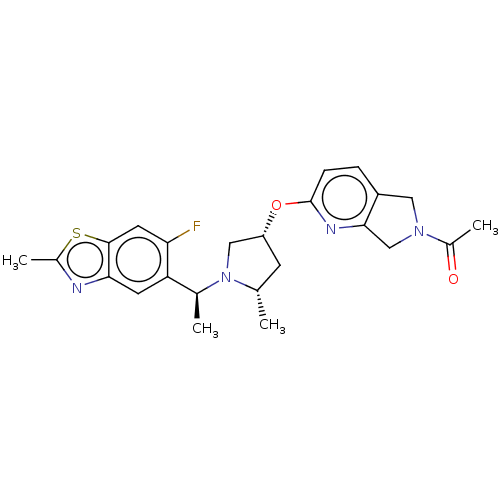
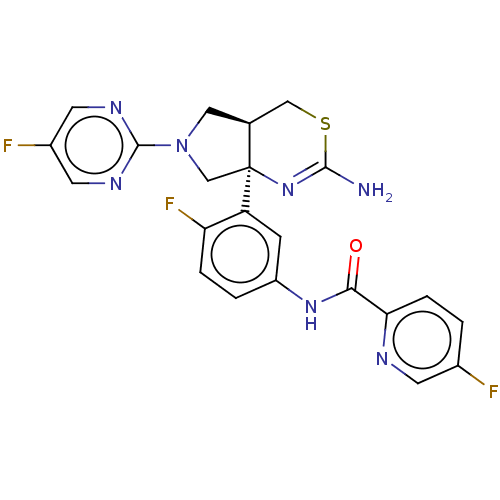
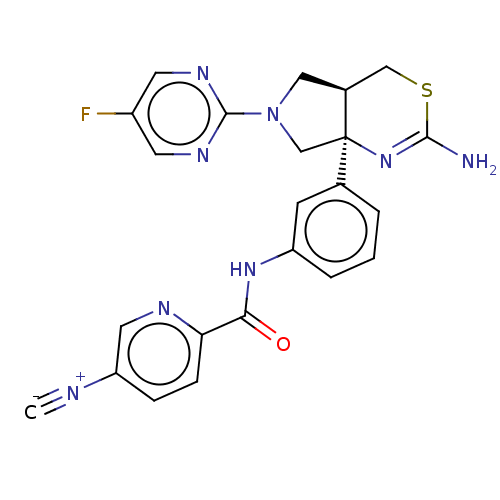
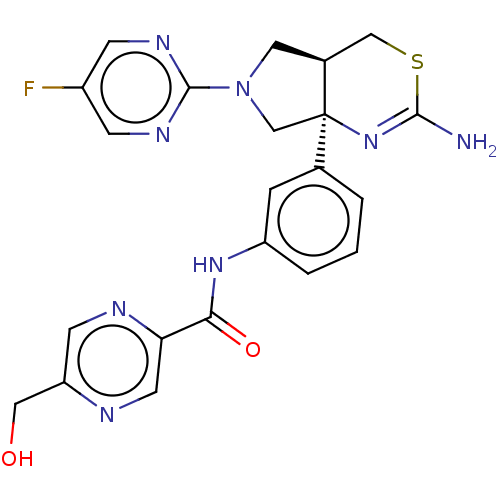
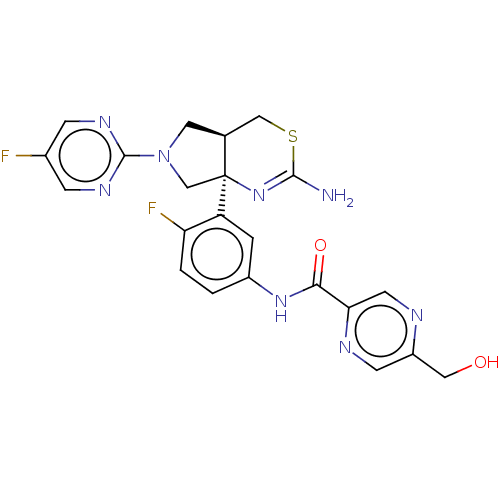
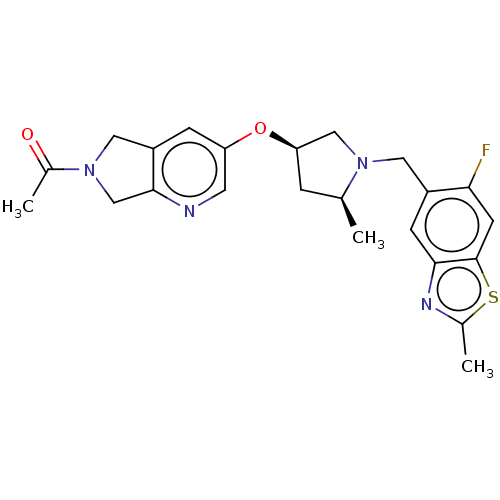
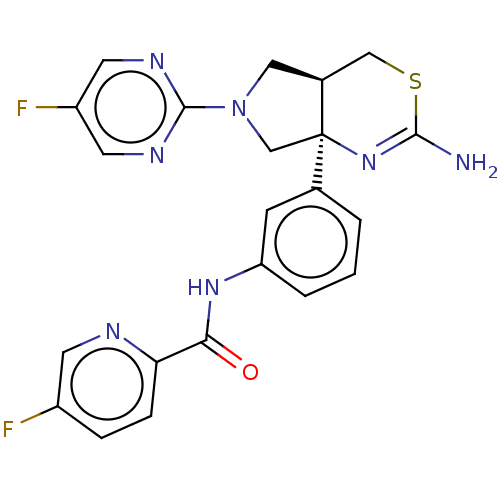
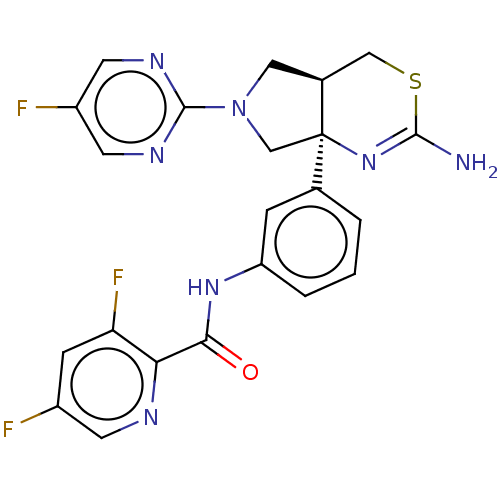
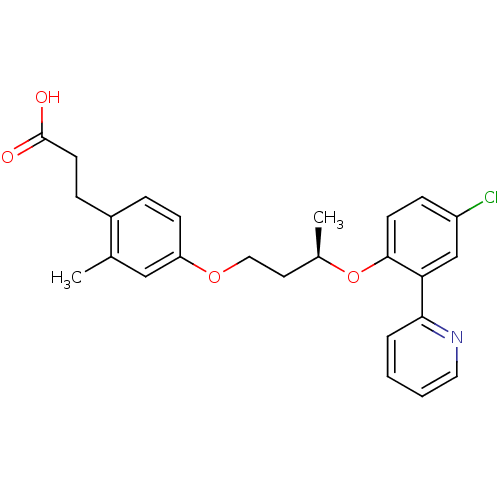
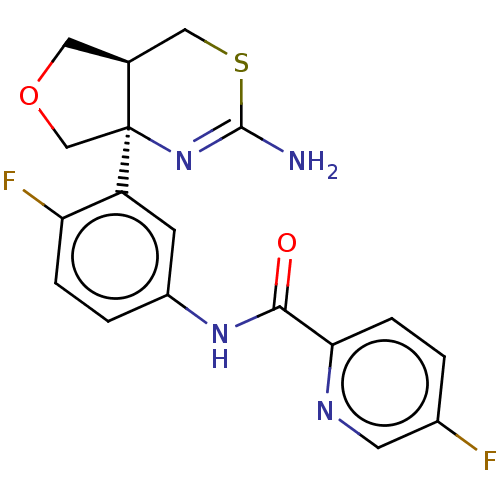
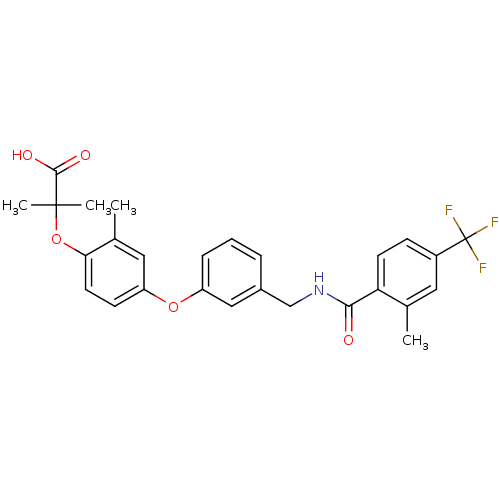
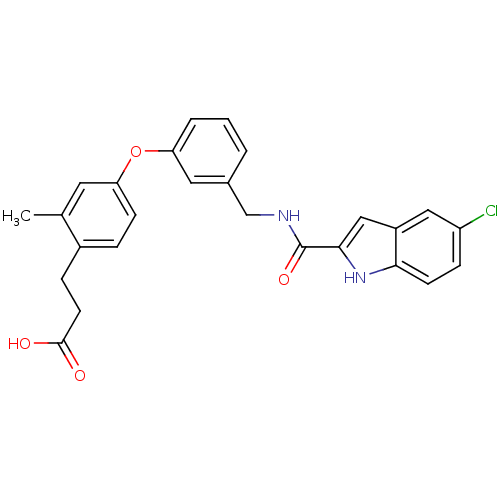
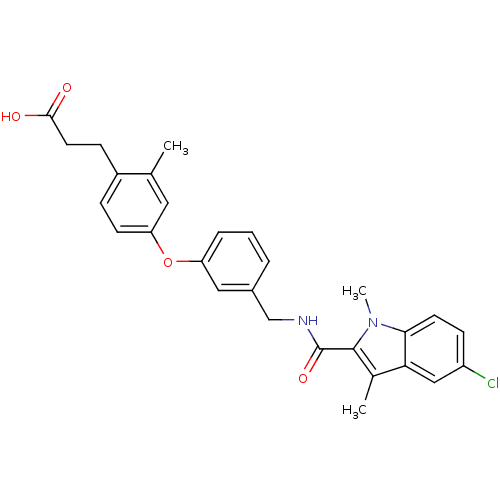
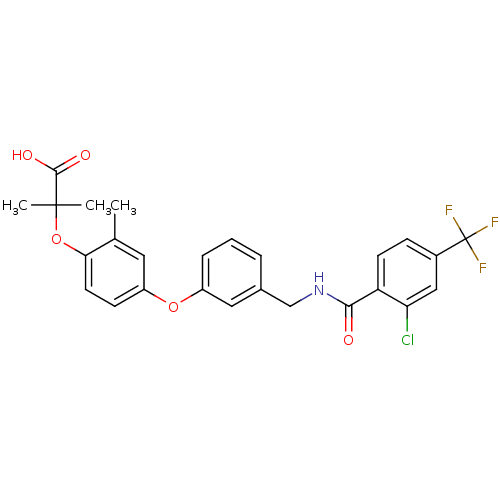
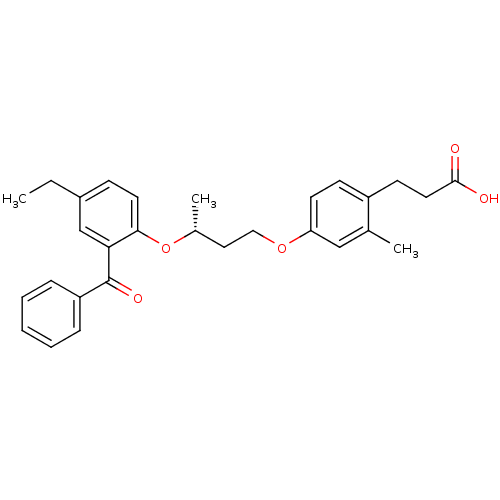
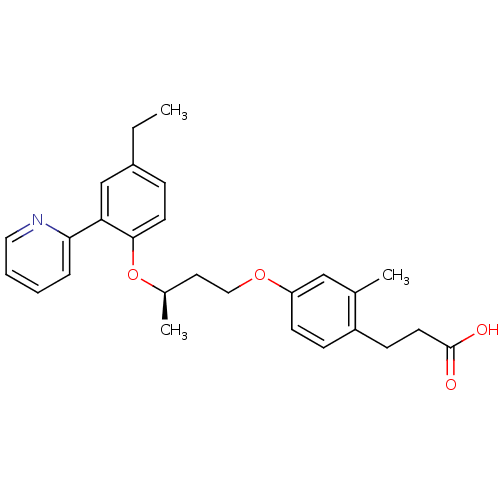
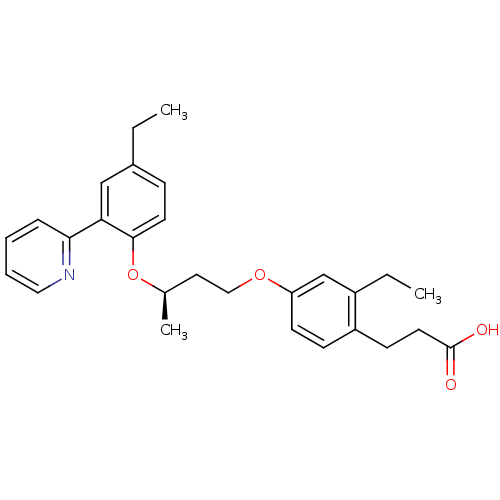
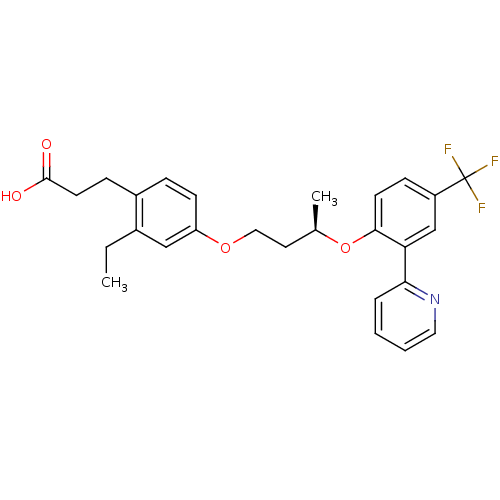
Affinity DataIC50: 0.214nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.214nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.275nMAssay Description:Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.309nMAssay Description:Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.358nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.385nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.385nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.388nMAssay Description:Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.465nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.465nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.481nMAssay Description:Inhibition of BACE1 in mouse primary cortical neuron assessed as reduction in Amyloid-beta level incubated for 24 hrs by sandwich ELISA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.482nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.495nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.495nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.554nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.555nMAssay Description:Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.569nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.592nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.592nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.603nMAssay Description:Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.610nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.615nMAssay Description:Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.730nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.739nMpH: 4.6 T: 2°CAssay Description:Serial dilutions of test compounds are prepared as described above. Compounds are further diluted 20x in KH2PO4 buffer. Ten uL of each dilution is ad...More data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.782nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.782nMAssay Description:The OGA enzyme catalyses the removal of O-GlcNAc from nucleocytoplasmic proteins. To measure this activity Fluorescein di-N-acetyl-β-N-acetyl-D-...More data for this Ligand-Target Pair
Affinity DataIC50: 0.871nMAssay Description:Inhibition of recombinant human BACE2 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate by FRET assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of src kinaseMore data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma(Homo sapiens (Human))
Lilly Spain S.A.
Curated by ChEMBL
Lilly Spain S.A.
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbINGMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibition of recombinant human BACE1 using (MCA)-S-E-V-N-L-D-A-E-F-R-K(dinitrophenol)-R-R-R-R-NH2 as substrate incubated for 8 hrs by FRET assayMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]-2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl butyric acid from human PPARdeltaMore data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor delta(Homo sapiens (Human))
Lilly Research Laboratories
Curated by ChEMBL
Lilly Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Displacement of [3H]2-(4-{2-[3-(2,4-difluoro-phenyl)-1-heptyl-ureido]-ethyl}-phenoxy)-2-methyl-butyric acid from human PPARdelta by SPAMore data for this Ligand-Target Pair

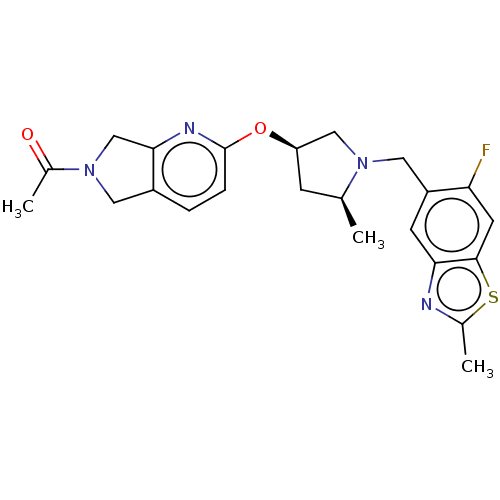
 3D Structure (crystal)
3D Structure (crystal)