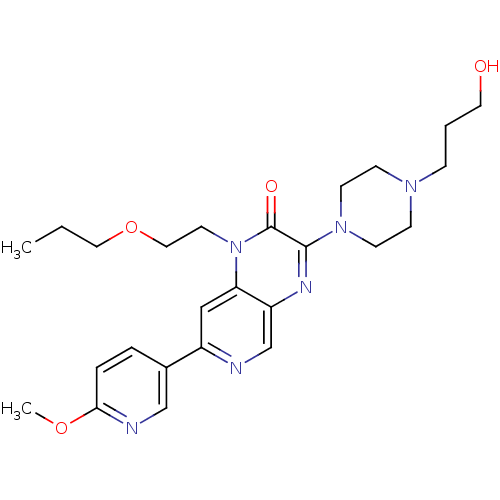
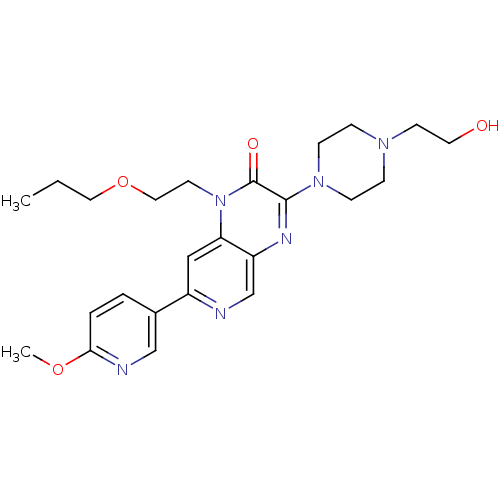
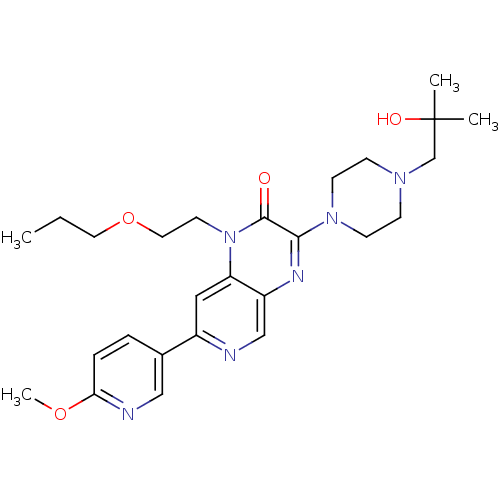
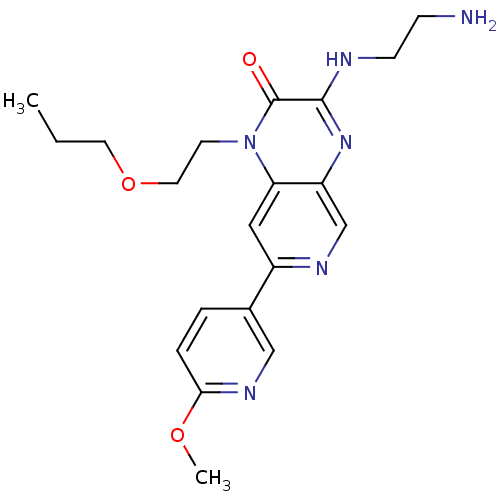
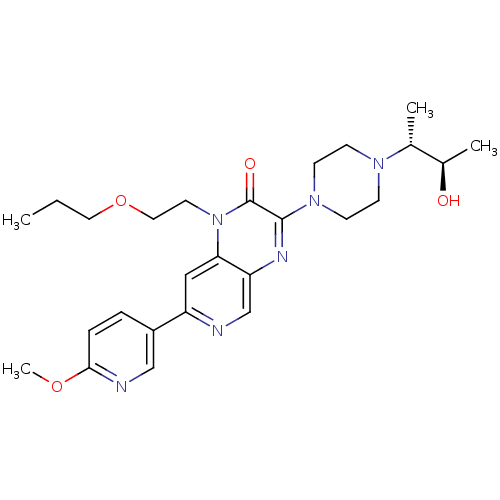
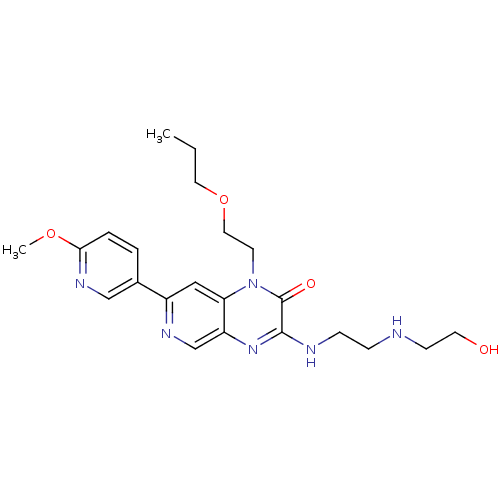
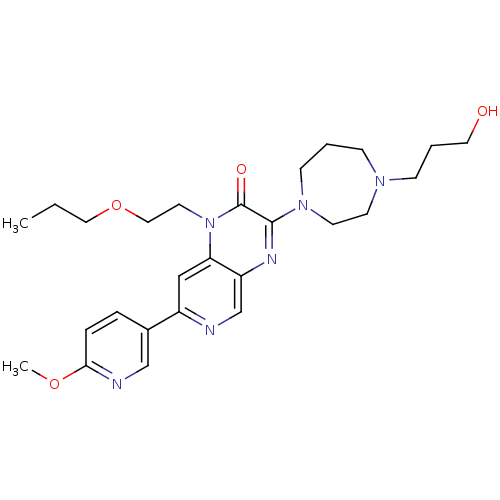
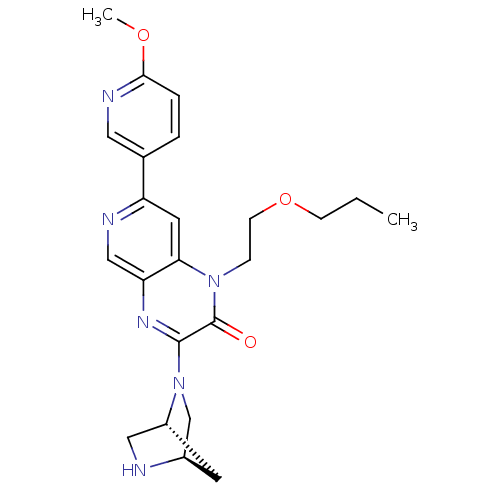
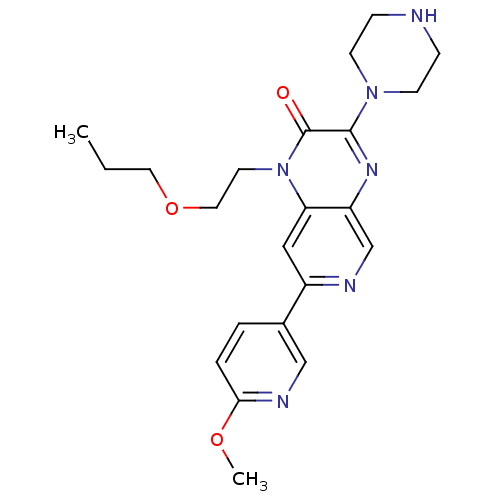
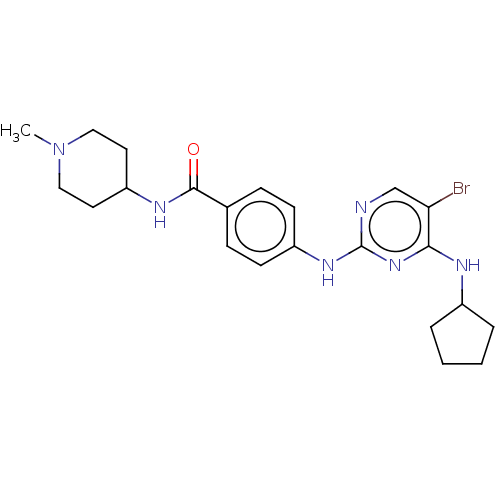
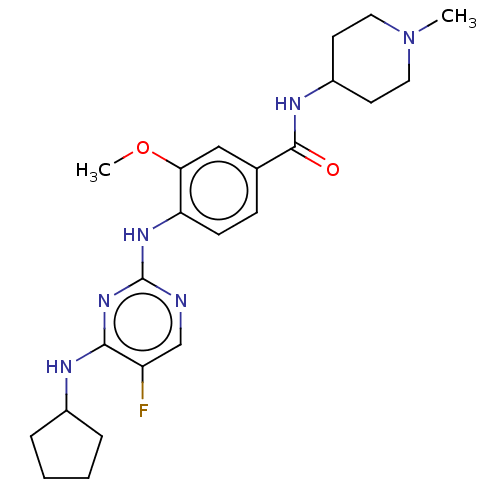
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.180nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.220nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.25nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.310nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.330nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 0.720nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Inhibition of human platelet PDE5 assessed as reduction in conversion of [3H]cGMP to [3H]GMP after 2 hr by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of Aurora A in human HeLa cells after 12 hrs by ELISA methodMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of Aurora A in human HeLa cells after 12 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 54nMAssay Description:Inhibition of human ERG by patch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Inhibition of human ERG by patch-clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 261nMAssay Description:Inhibition of Aurora A in human HeLa cells after 12 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of human ERG by patch-clamp methodMore data for this Ligand-Target Pair
Affinity DataIC50: 395nMAssay Description:Inhibition of Aurora B in human HeLa cells after 12 hrs by ELISA methodMore data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of Aurora B in human HeLa cells after 12 hrs by ELISA methodMore data for this Ligand-Target Pair
Affinity DataIC50: 453nMAssay Description:Inhibition of Aurora B in human HeLa cells after 12 hrs by ELISA methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 670nMAssay Description:Inhibition of human ERG by patch-clamp methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 1.79E+3nMAssay Description:Inhibition of human ERG by patch-clamp methodMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE4BMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4A(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE4AMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE3BMore data for this Ligand-Target Pair
TargetcGMP-inhibited 3',5'-cyclic phosphodiesterase 3A(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE3AMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE1CMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE1BMore data for this Ligand-Target Pair
TargetDual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE1AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4C(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE4CMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4D(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE4DMore data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE8BMore data for this Ligand-Target Pair
TargetHigh affinity cAMP-specific and IBMX-insensitive 3',5'-cyclic phosphodiesterase 8A(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE8AMore data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 7B(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PDE7BMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of human ERG by patch-clamp methodMore data for this Ligand-Target Pair