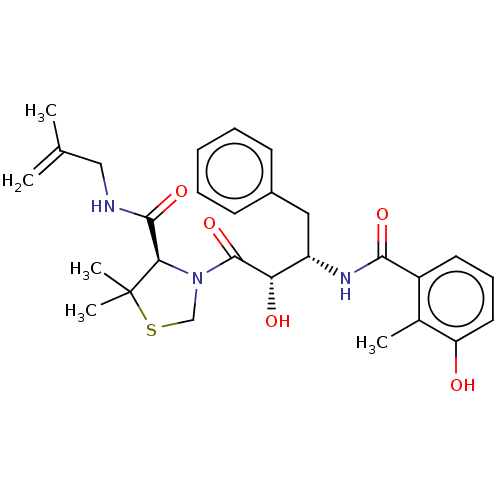
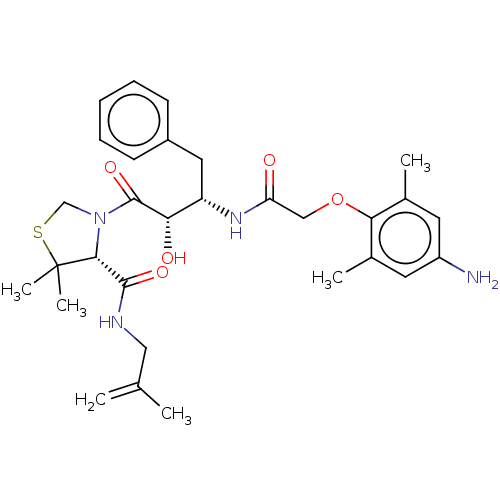
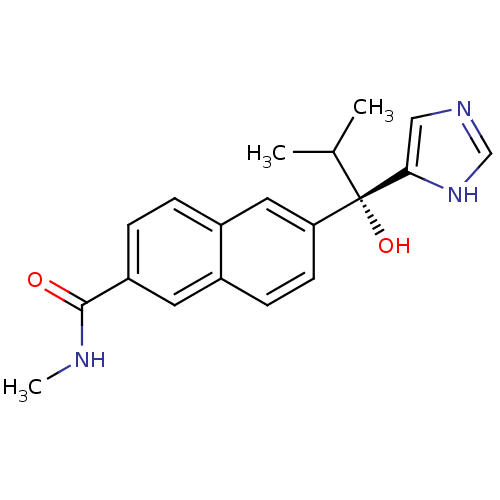
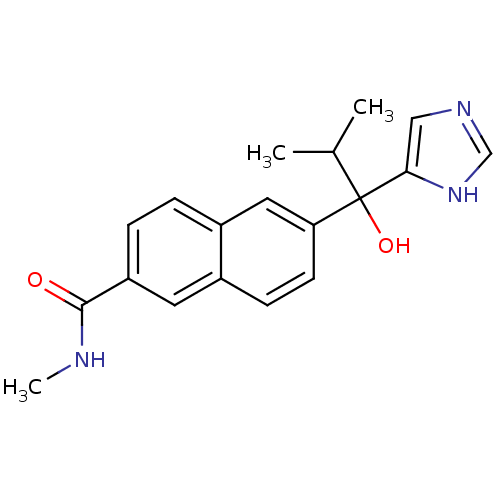
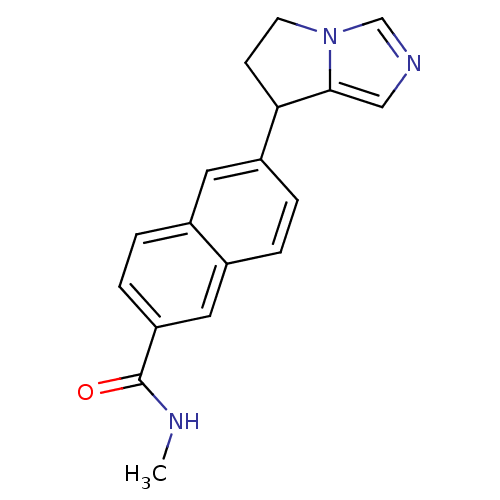
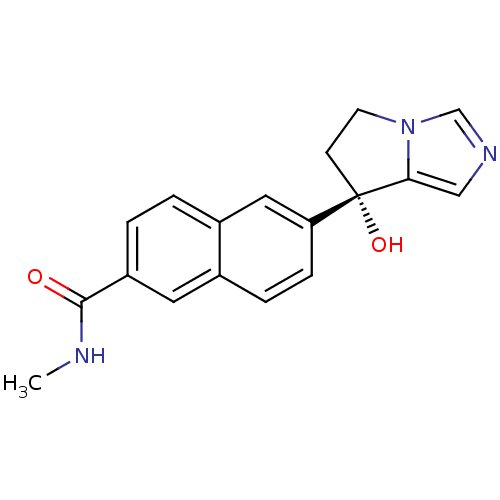
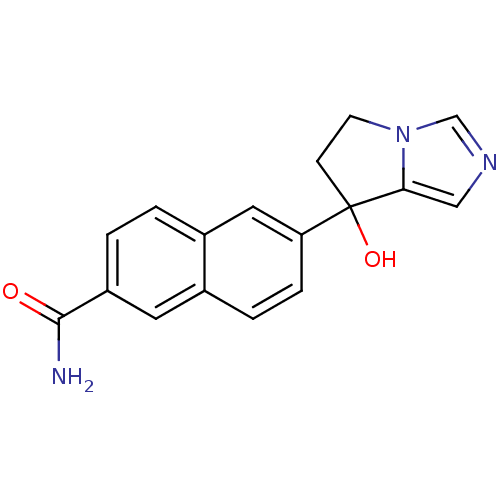
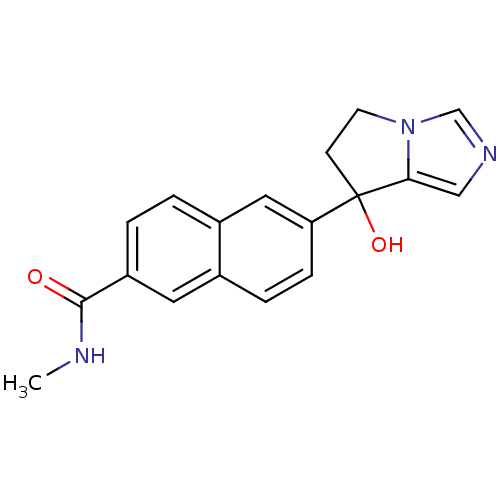
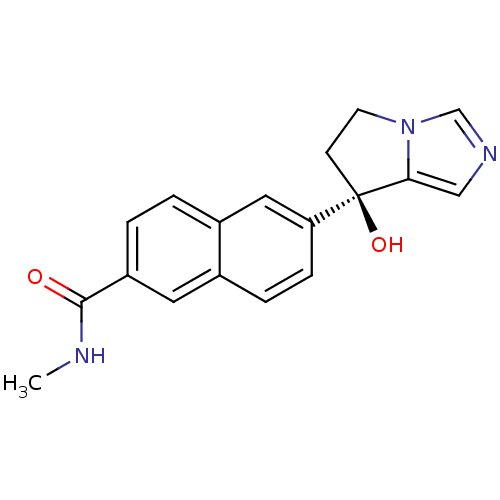
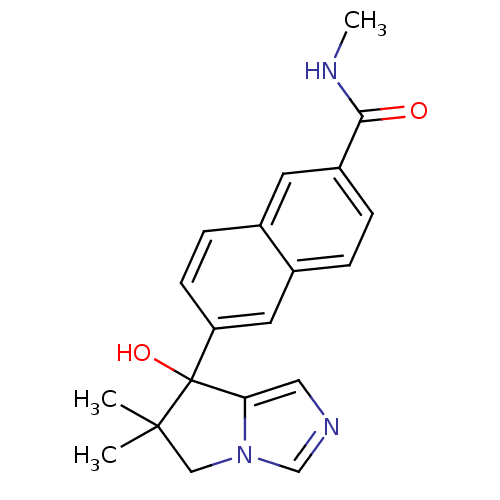
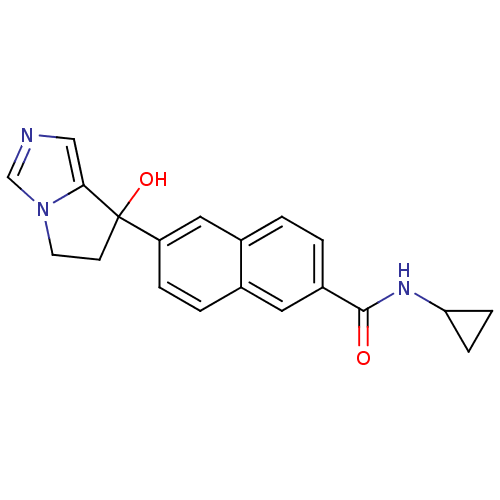
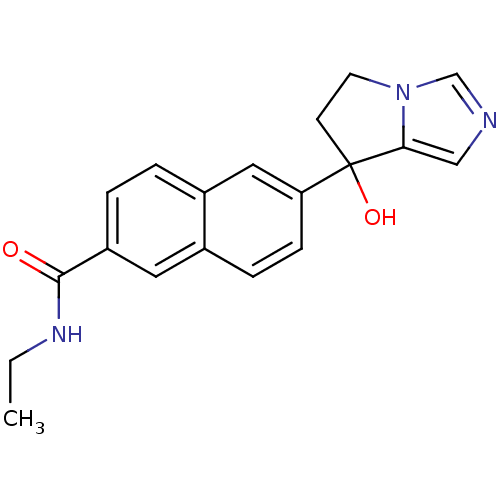
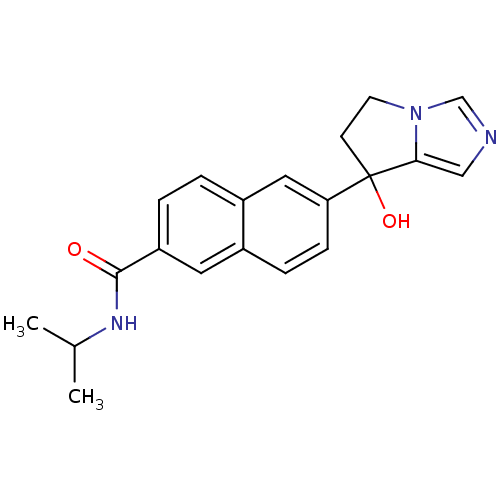
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 0.00240nMAssay Description:Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
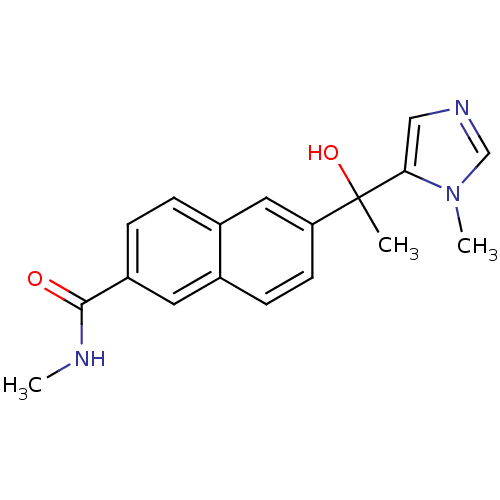
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 0.0310nMAssay Description:Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
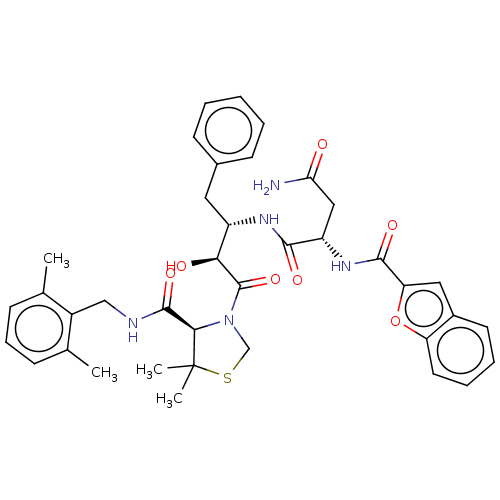
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 0.190nMAssay Description:Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
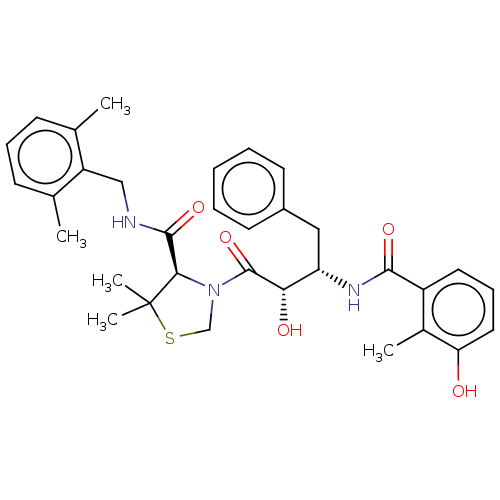
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataKi: 0.830nMAssay Description:Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 15nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 54nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 88nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 160nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 410nMAssay Description:Inhibition of 17,20-lyase activity of human CYP17A1More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4 assessed as conversion of testosterone into 6-hydroxytestosterone after 30 mins by HPLC analysisMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C19 assessed as (S)-mephenytoin 4'-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP1A2 assessed as 7-ethoxyresorufin O-deethylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2E1 assessed as 4-nitrophenol hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2D6 assessed as (+)-bufuralol 1'-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C9 (Arg) assessed as Tolbutamide hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C8 assessed as Tolbutamide hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2B6 assessed as ethoxycoumarin O-deethylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP3A4 assessed as testosterone 6beta-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2A6 assessed as coumarin 7-hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human B-lymphoblastoid cell microsomal CYP2C9 (Arg) assessed as Tolbutamide hydroxylation preincubated for 5 mins with substrateMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Kyoto Pharmaceutical University
Curated by ChEMBL
Kyoto Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 61nMAssay Description:Inhibition of protease L10F/V32I/M46I/I47V/Q58E/I84V mutant in HIV1 A17 infected in human MT4 cells assessed as reduction in virus-induced cytopathic...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)