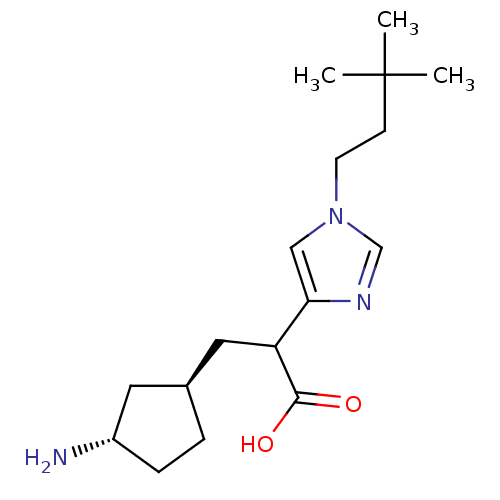
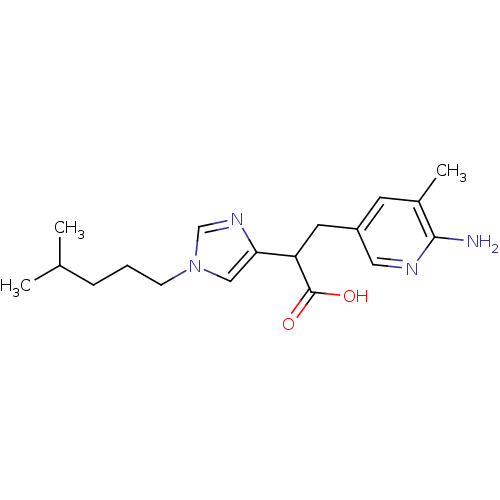
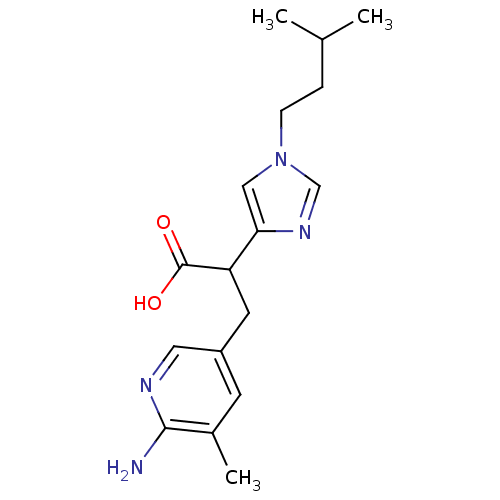
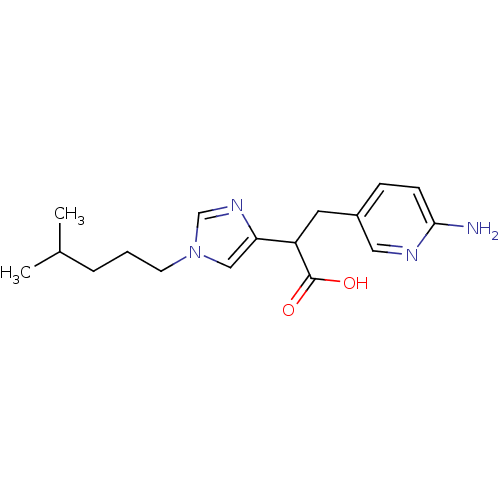
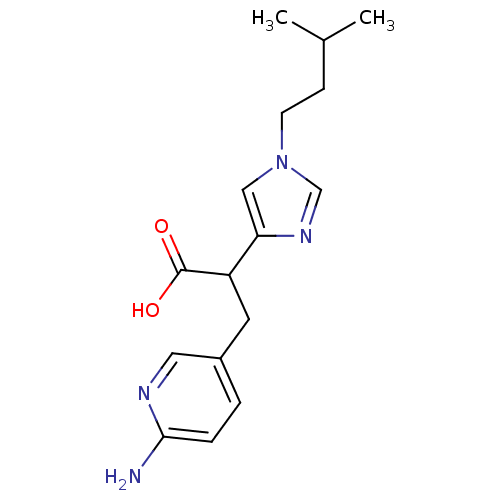
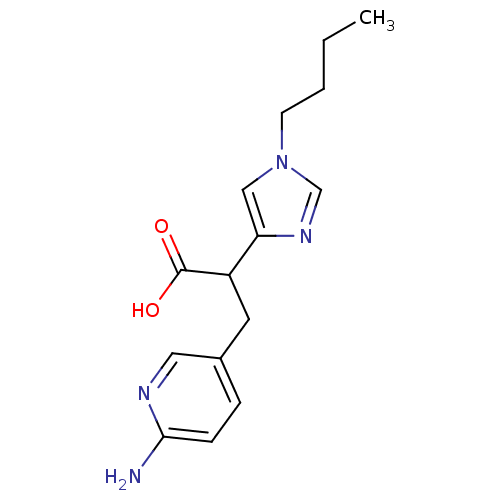
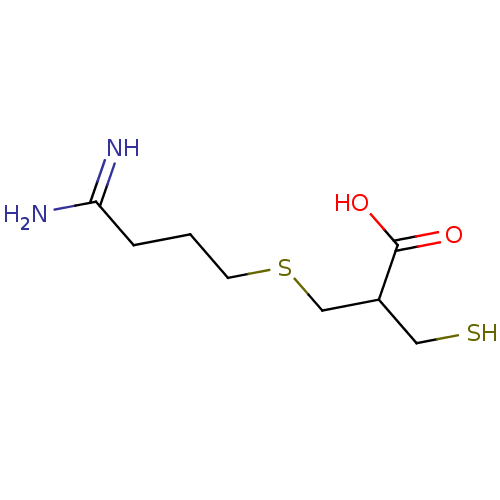
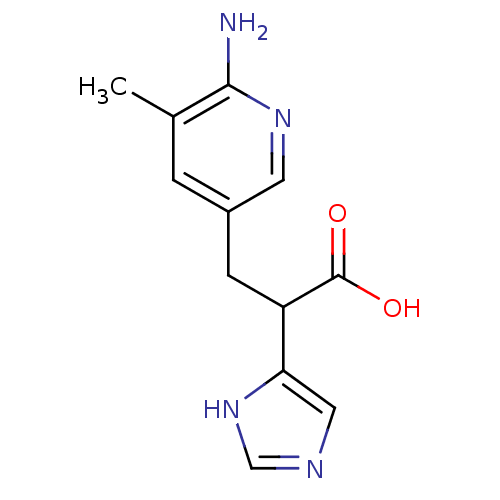
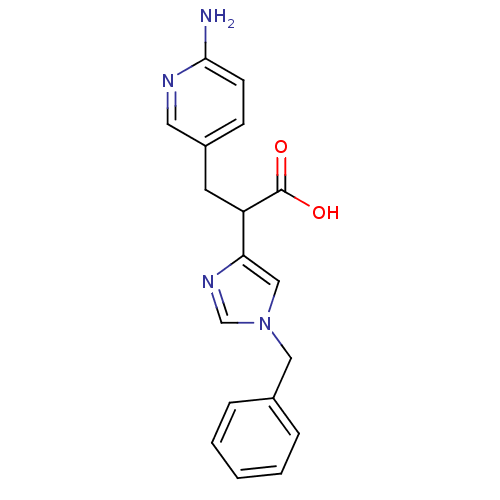
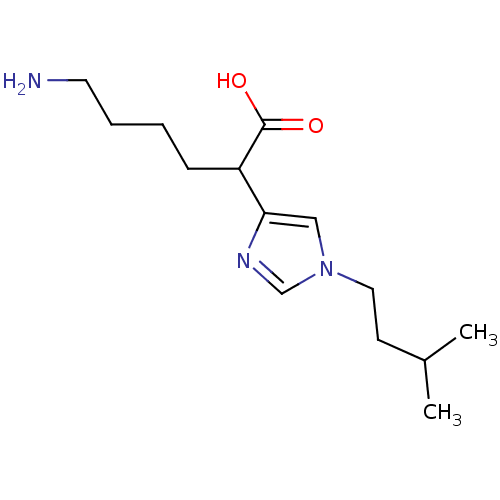
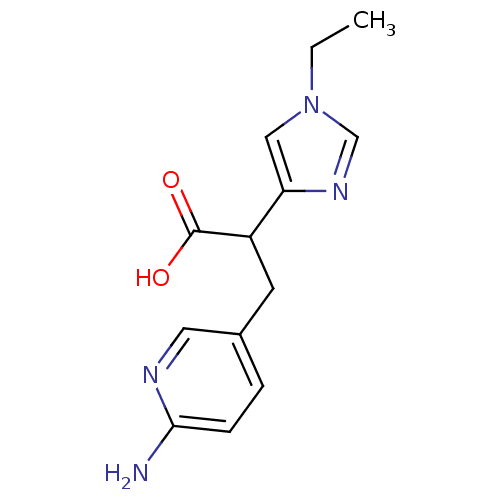
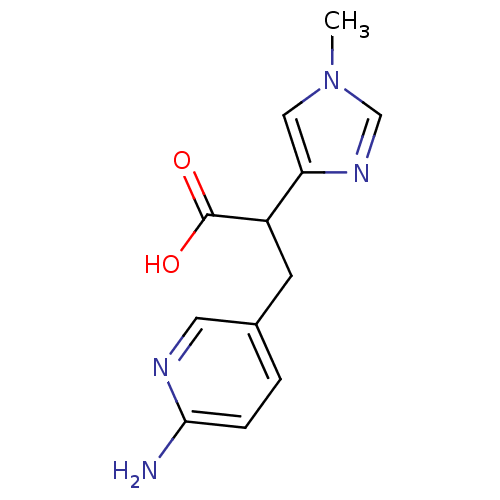
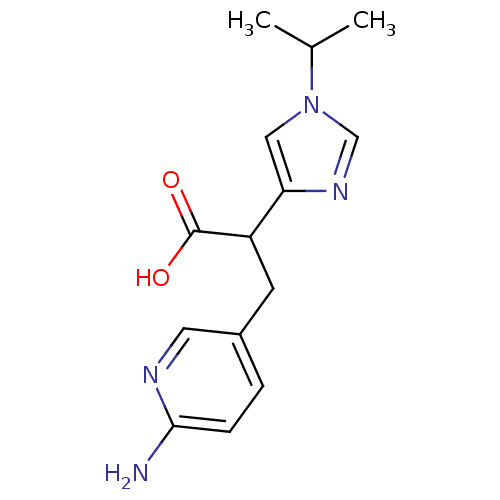
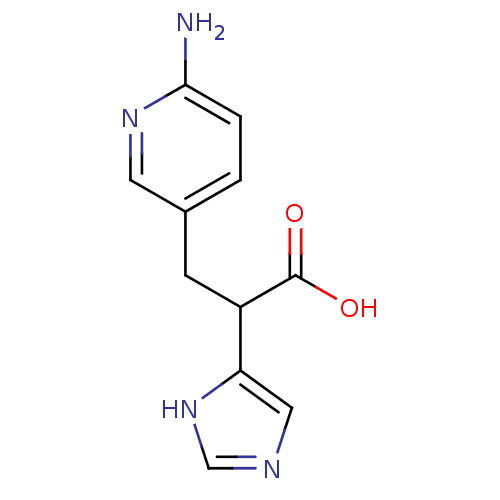
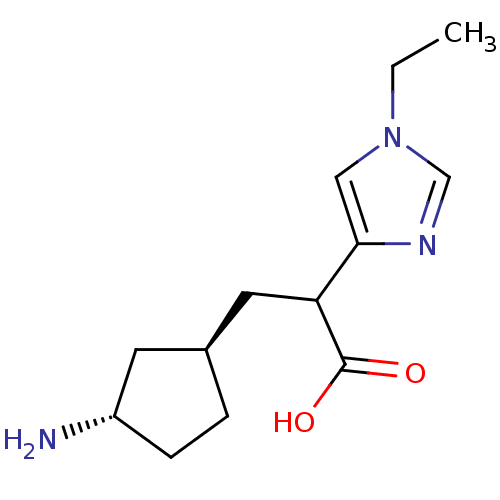
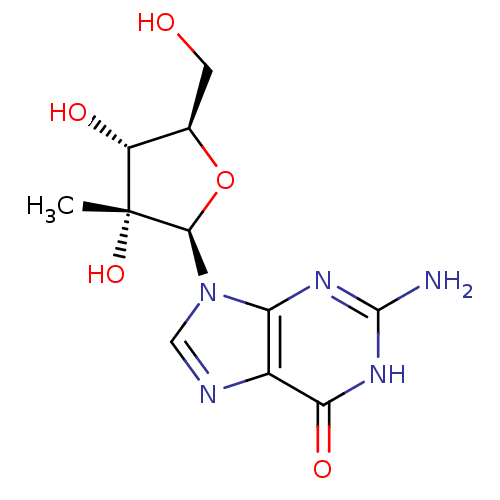
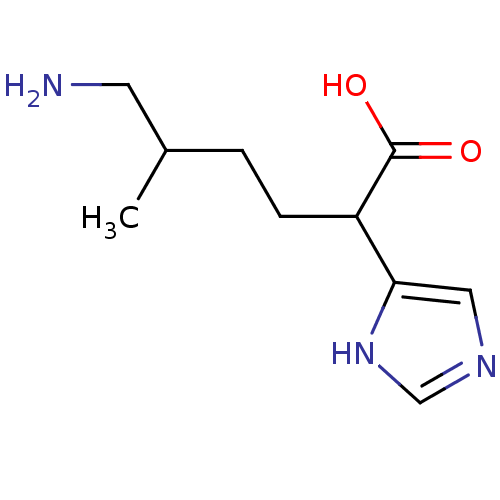
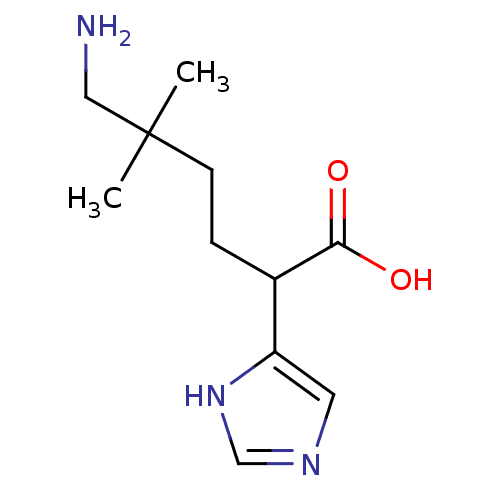
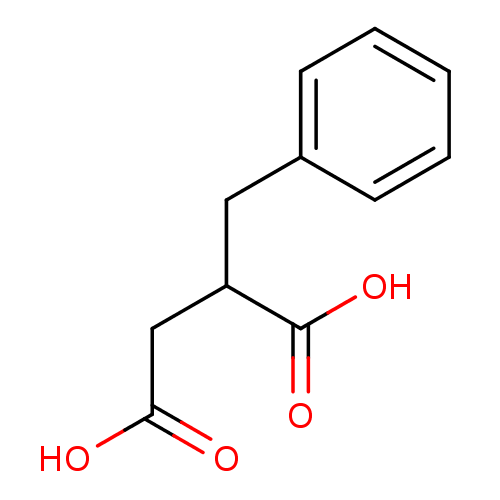
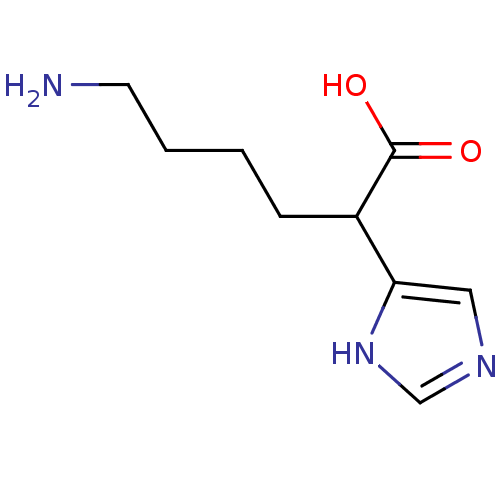
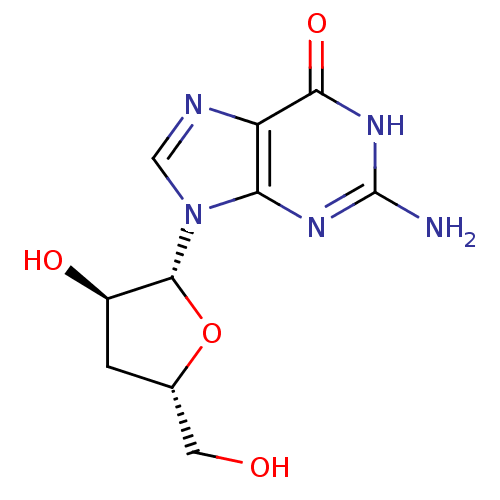
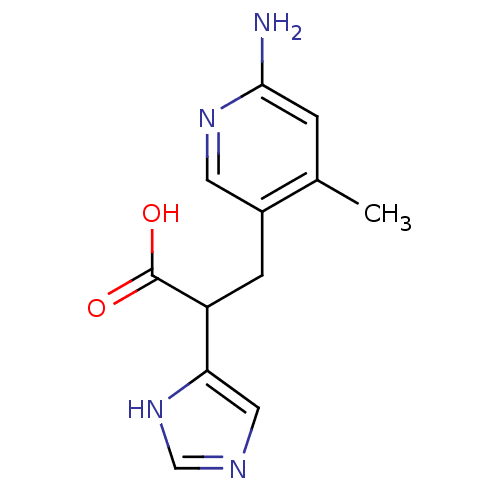
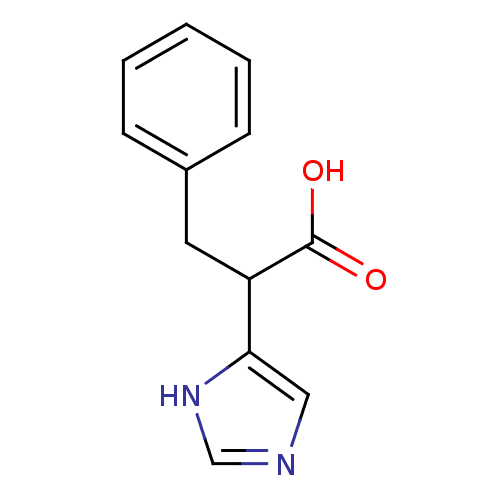
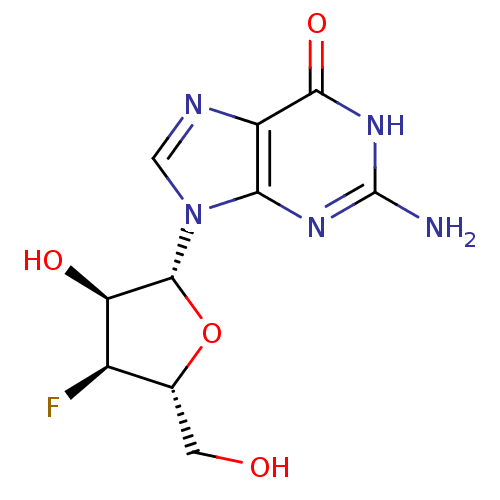
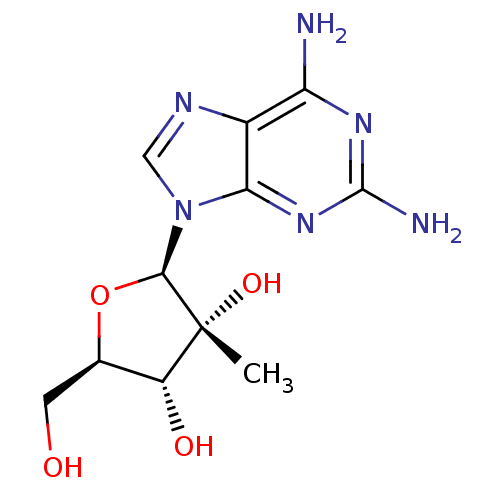
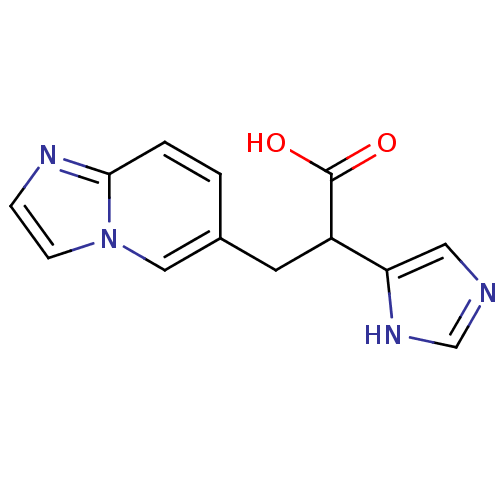
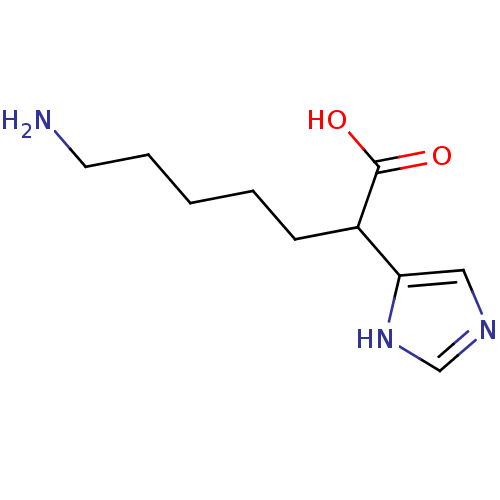
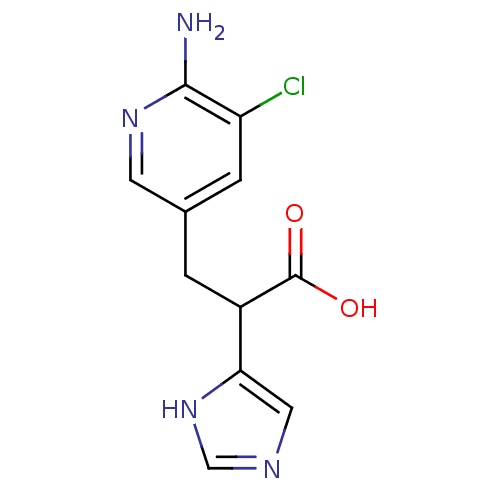
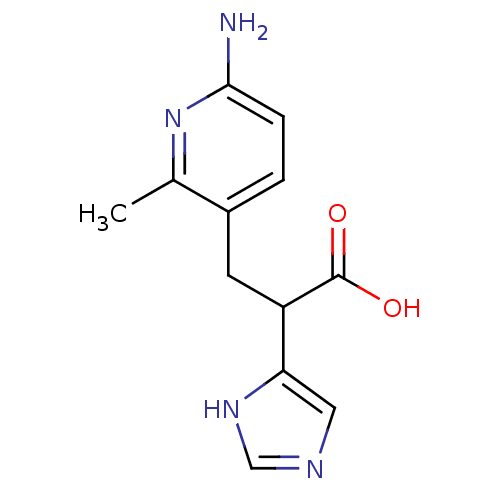
Affinity DataIC50: 1nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 8.20nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:In vitro inhibition of purified Carboxypeptidase M (CPM) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasmaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of human carboxypeptidase B (CPB)More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition HCV NS5B-mediated RNA synthesisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+3nMAssay Description:Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor)More data for this Ligand-Target Pair