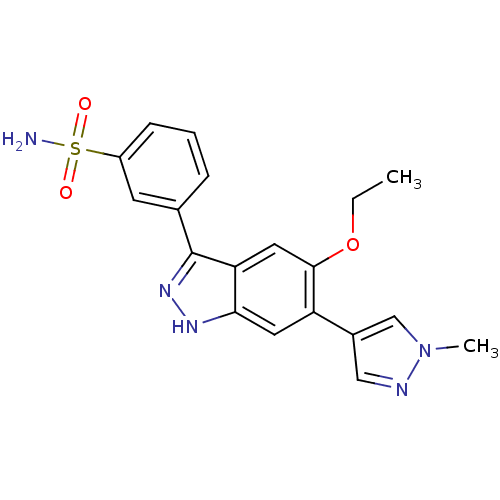
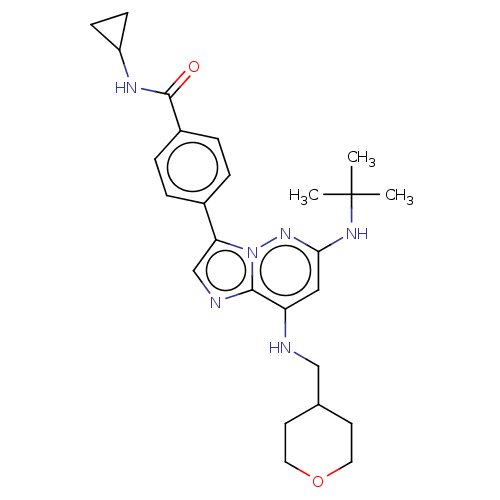
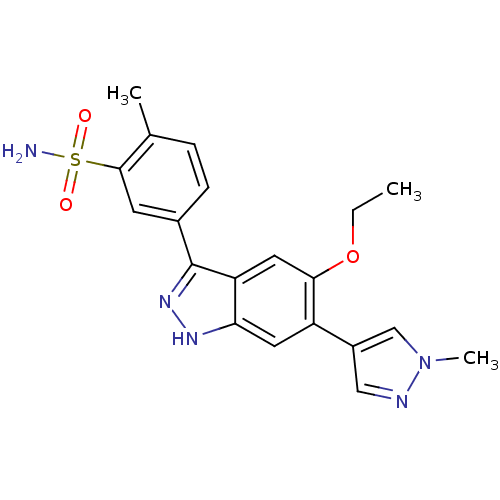
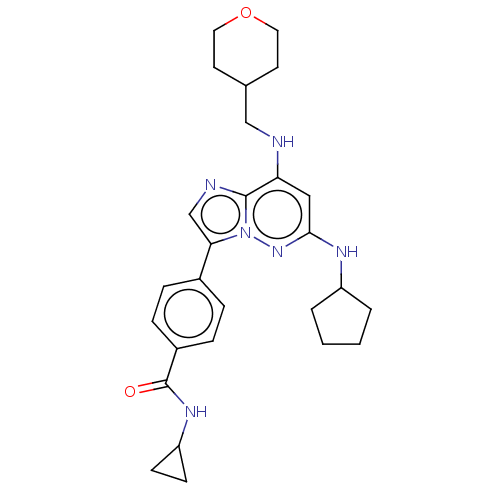
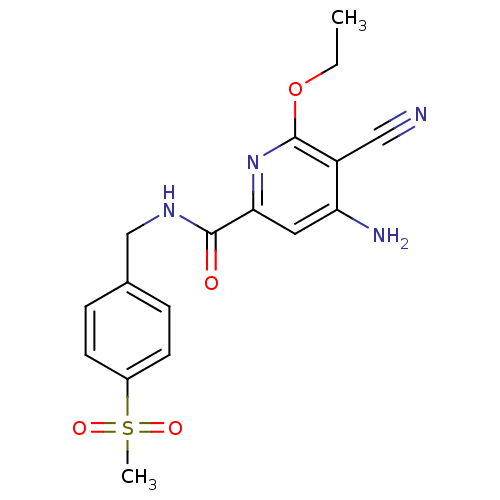
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin F2-alpha receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGF-2 from human Prostanoid FP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetThromboxane A2 receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]SQ-29,548 from human Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetThromboxane A2 receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]SQ-29,548 from human Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGD-2 from human Prostanoid DP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGD-2 from human Prostanoid DP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin F2-alpha receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGF-2 from human Prostanoid FP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 1.02E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.90E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP3 receptorMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Displacement of [3H]PGD-2 from human Prostanoid DP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 6.80E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP3 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 8.20E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP3 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: 9.60E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP3 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin F2-alpha receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGF-2 from human Prostanoid FP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP3 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetThromboxane A2 receptor(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]SQ-29,548 from human Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Fujisawa Pharmaceutical Co., Ltd.
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human MPS1 expressed in Escherichia coliMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Inhibition of MPS1 (unknown origin) using biotin-labeled AGAGLARHTDDEMTGYVA as substrate after 90 mins by DELFIAMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of FLAG-tagged Mps1 autophosphorylation in human RERF-LC-AI cells expressing Tet-suppressible promotor after 3 hrs by immunoblottingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of JNK1-mediated ATF2 phosphorylation after 1 hr by ELISAMore data for this Ligand-Target Pair
TargetDual specificity protein kinase TTK(Homo sapiens (Human))
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Shionogi Pharmaceutical Research Center
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Inhibition of Mps1 (unknown origin)-mediated p38 MAPK phosphorylation after 90 mins by DELFIA assayMore data for this Ligand-Target Pair




 3D Structure (crystal)
3D Structure (crystal)