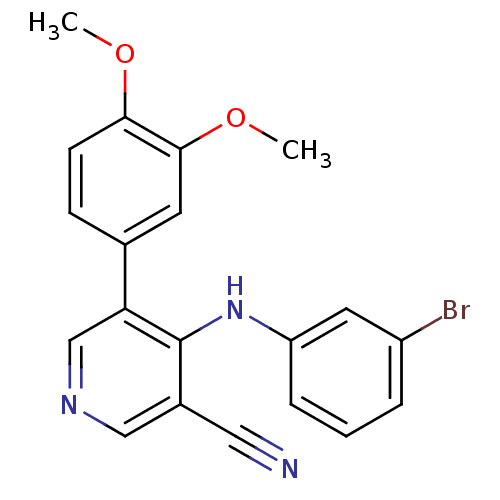
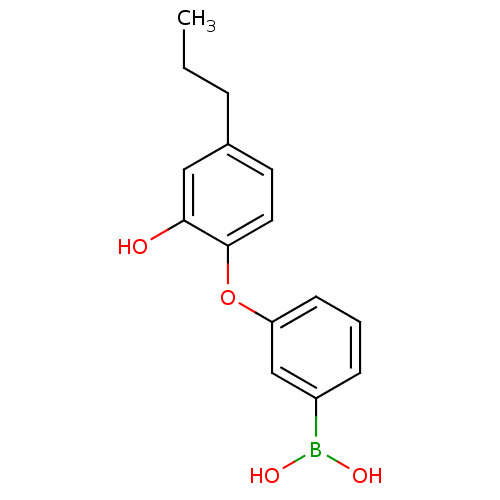
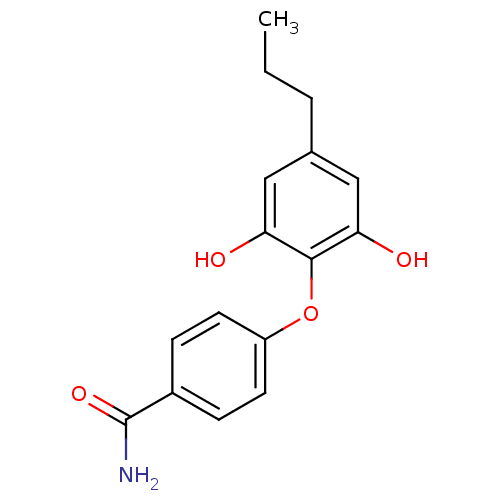
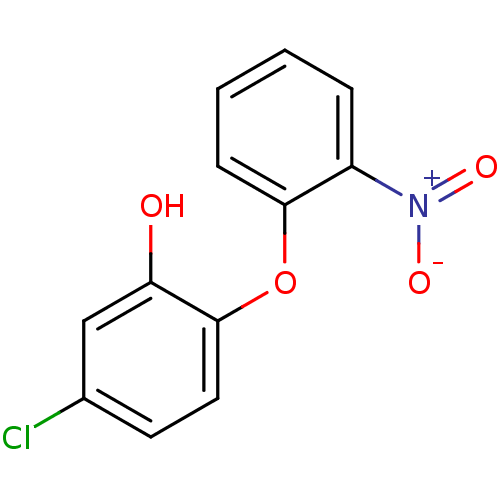
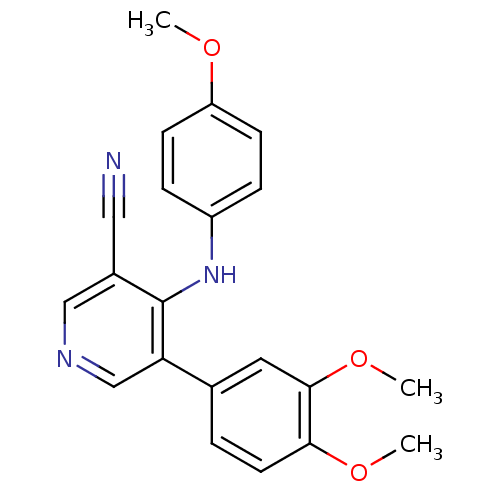
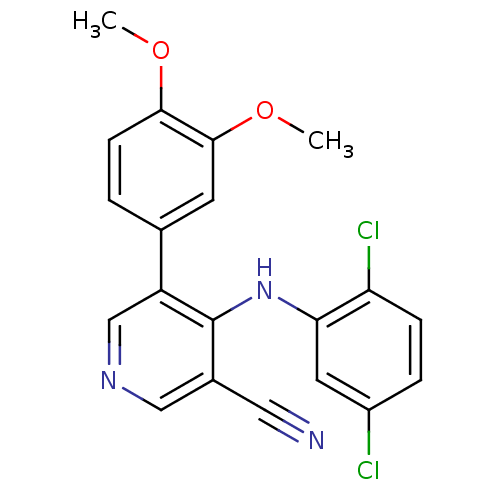
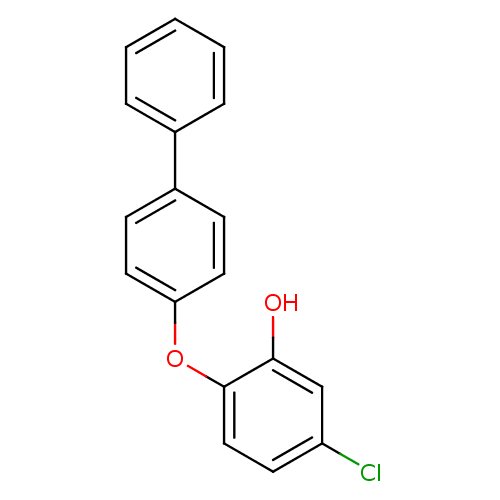
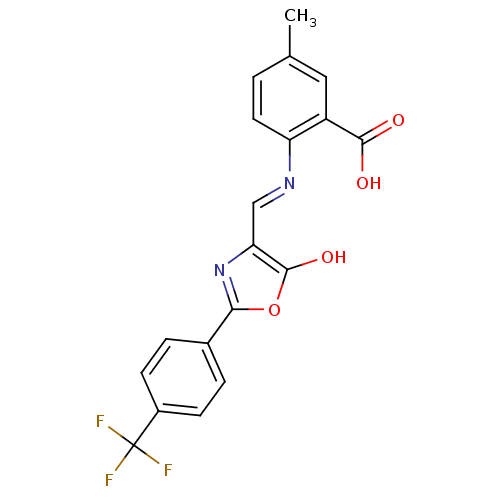
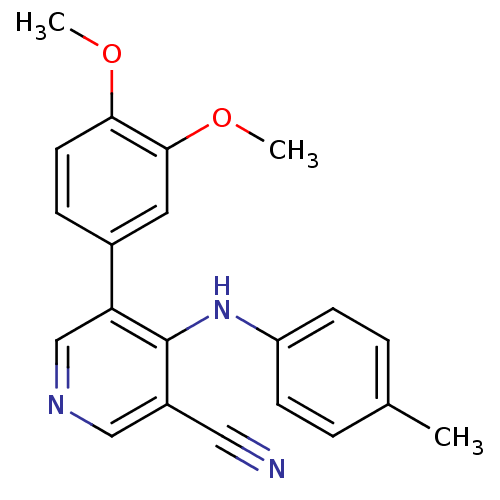
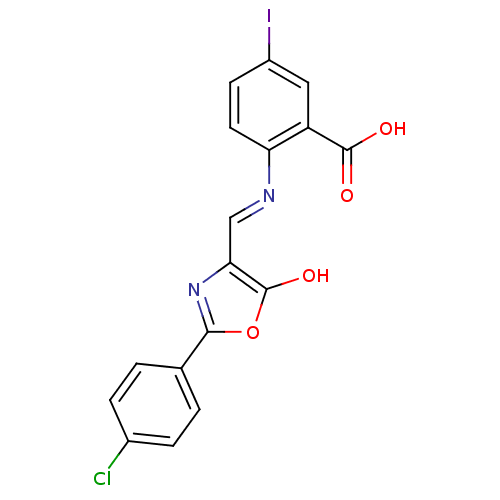
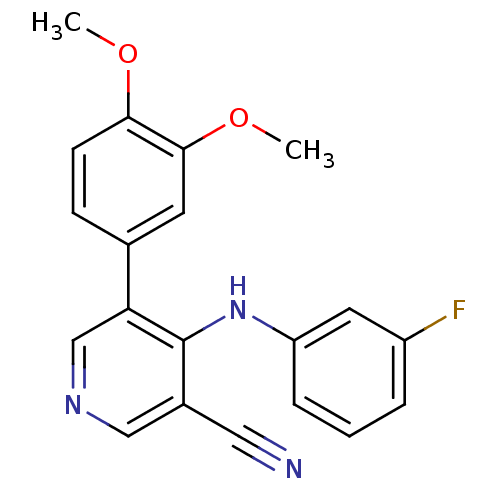
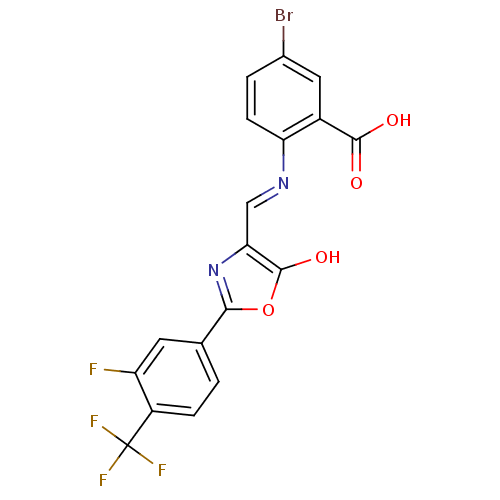
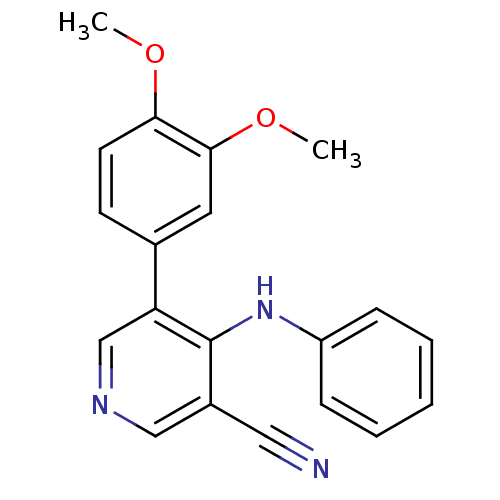
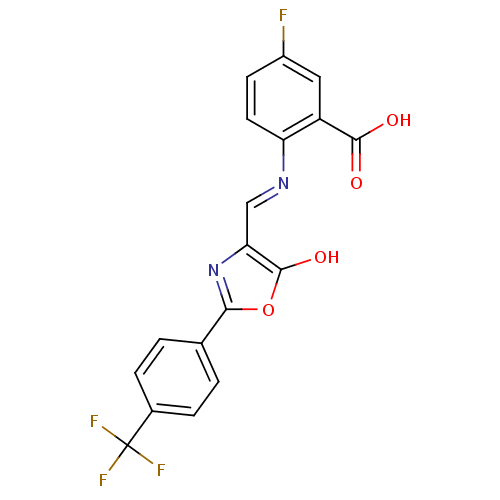
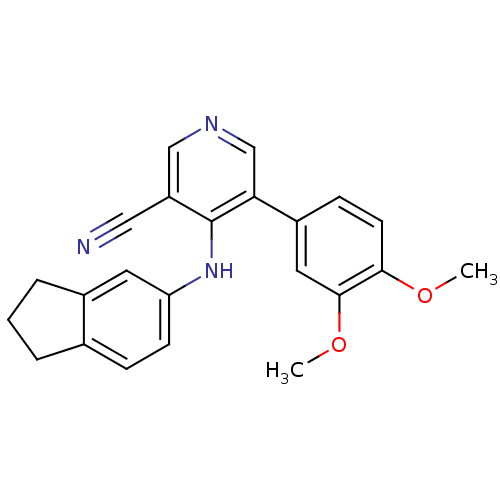
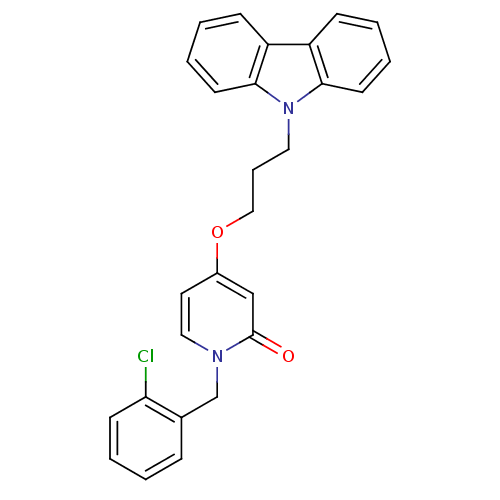
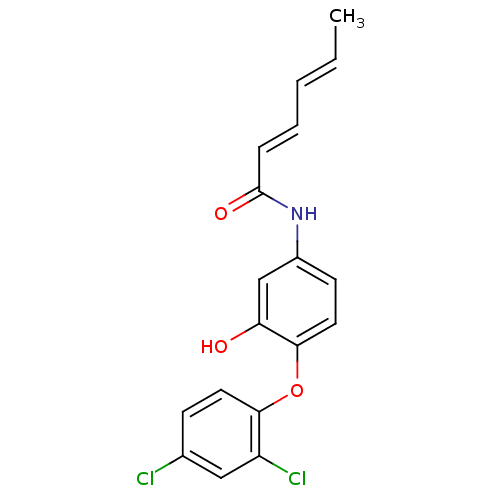
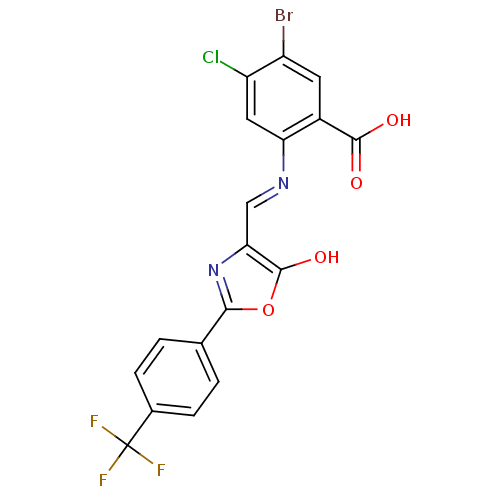
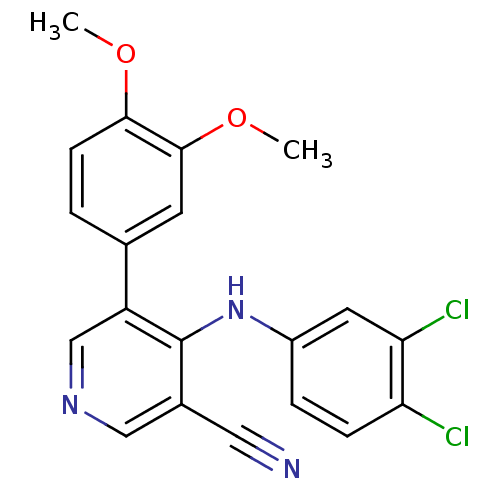
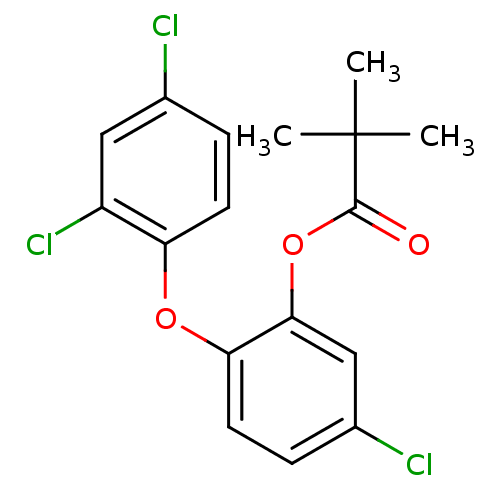
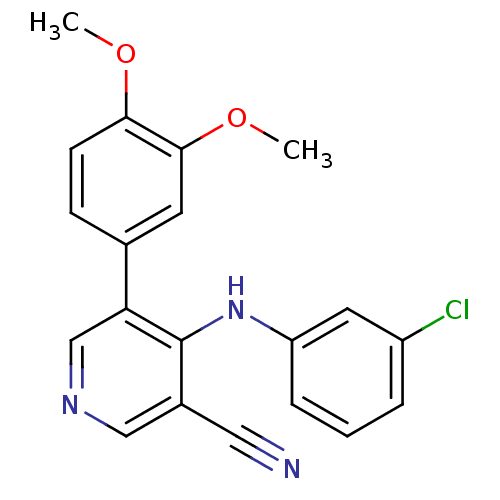
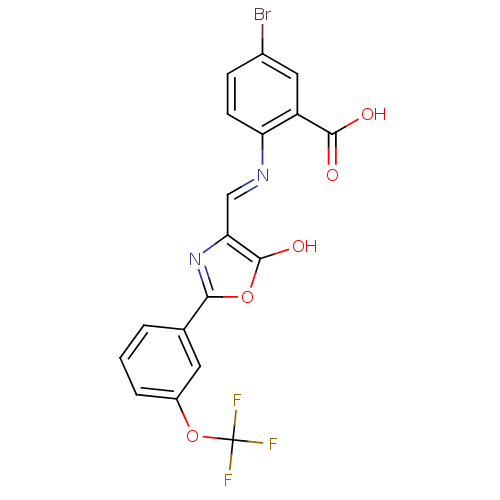
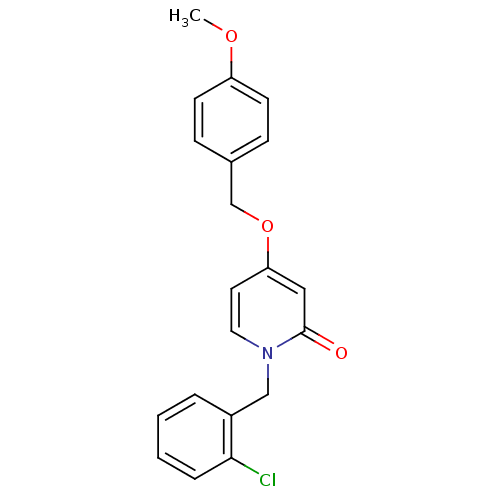
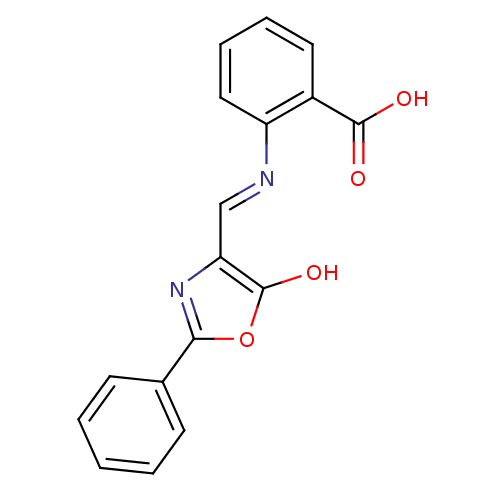
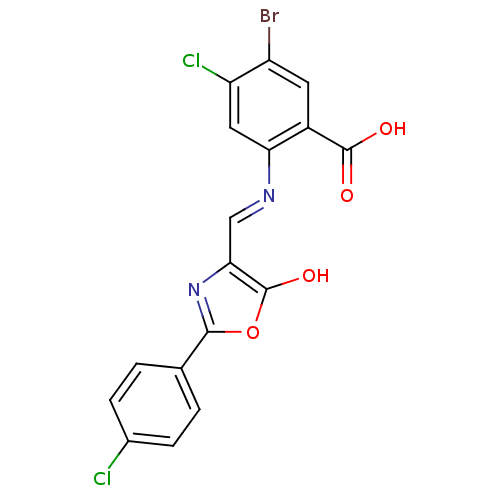
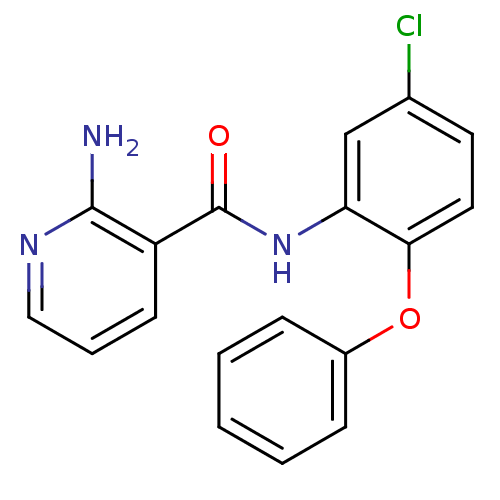
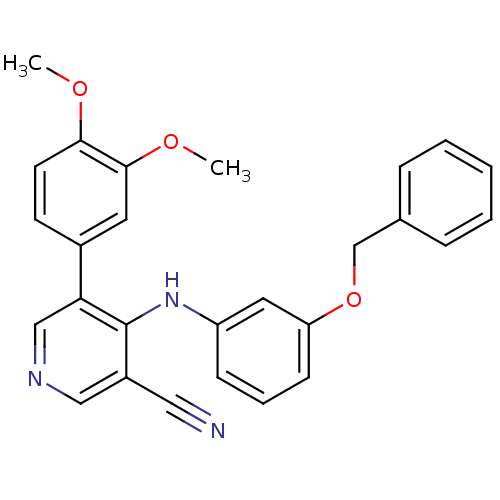
Affinity DataKi: 79nM ΔG°: -40.1kJ/mole IC50: 70nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
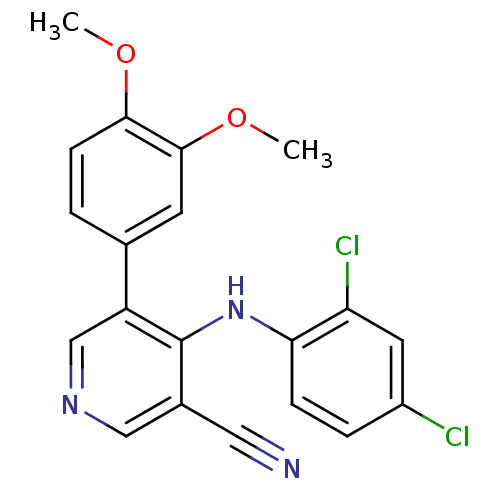
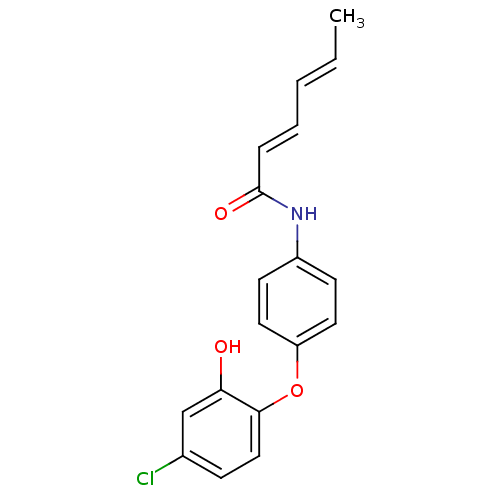
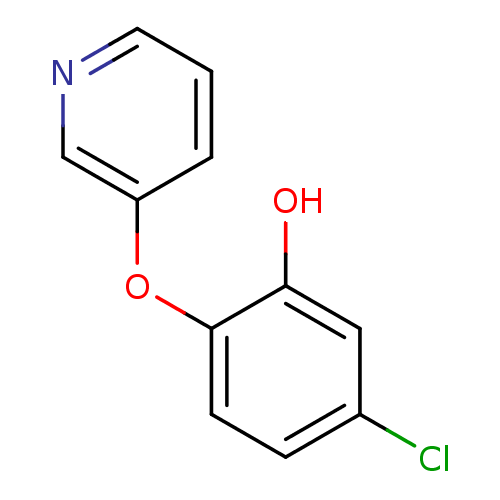
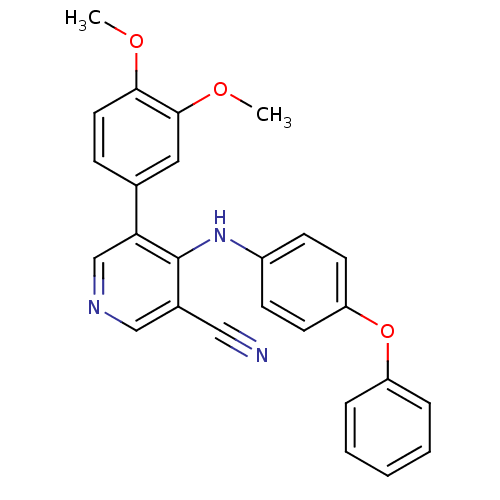
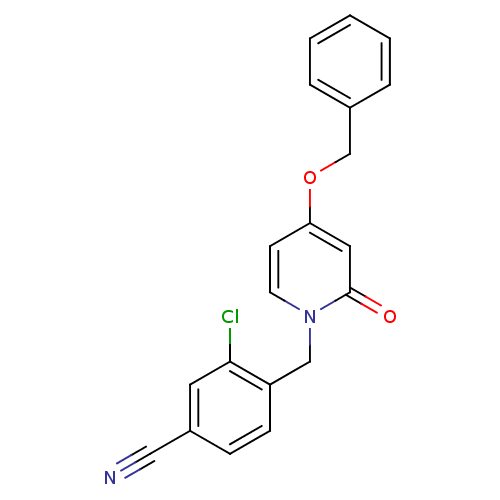
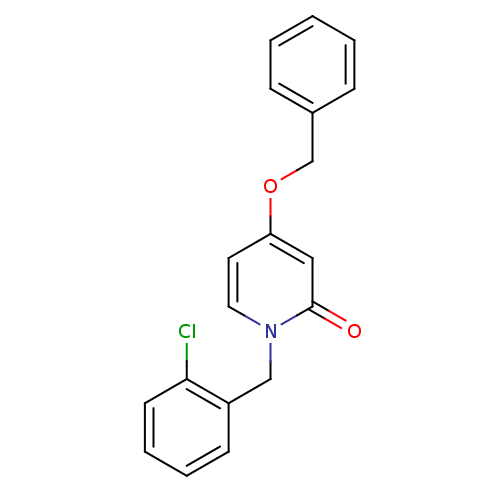
Affinity DataKi: 4.90E+3nM ΔG°: -30.0kJ/mole IC50: 4.60E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
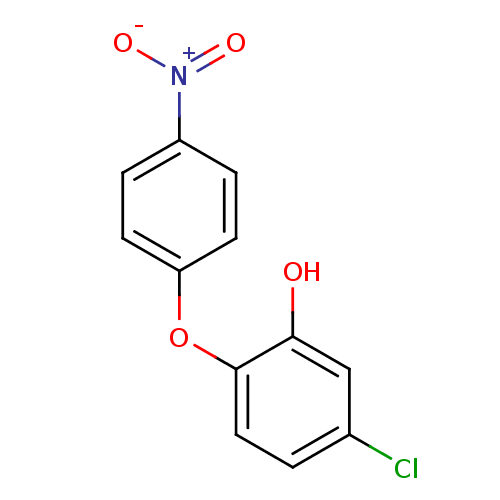
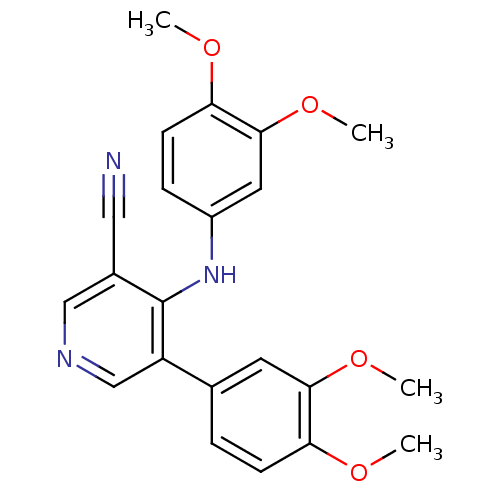
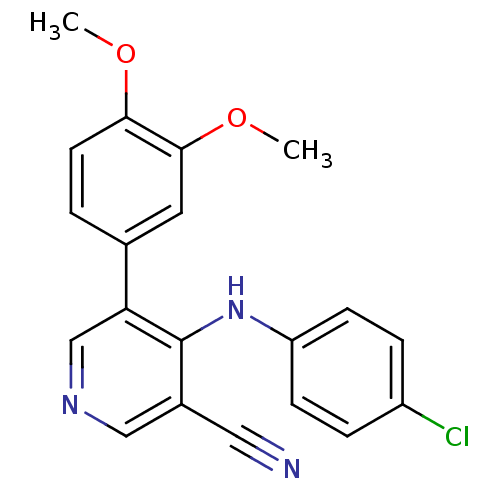
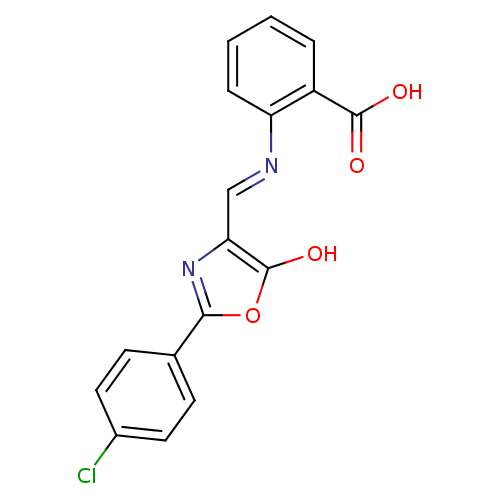
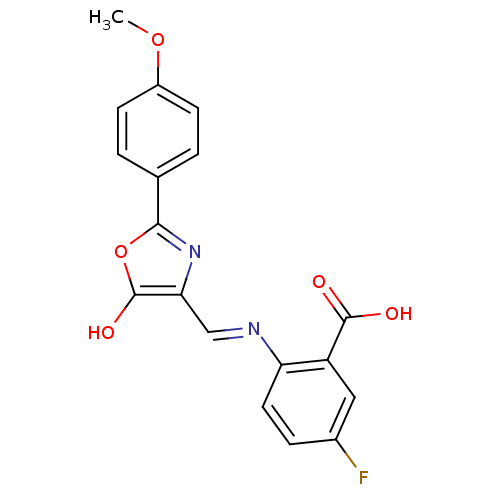
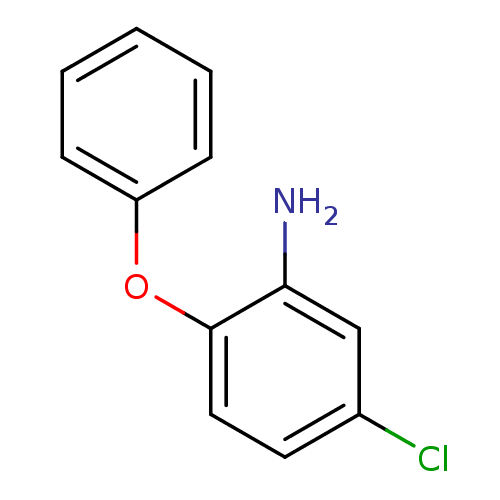
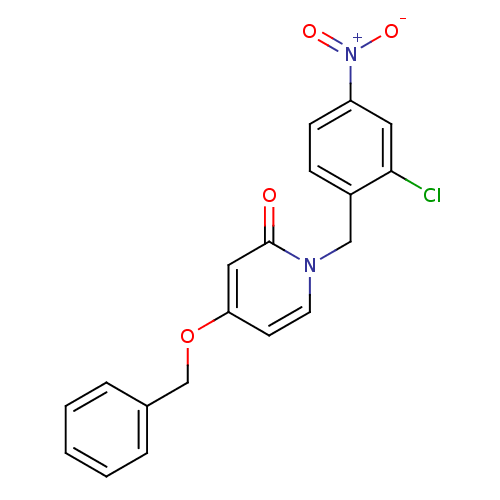
Affinity DataIC50: 80nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
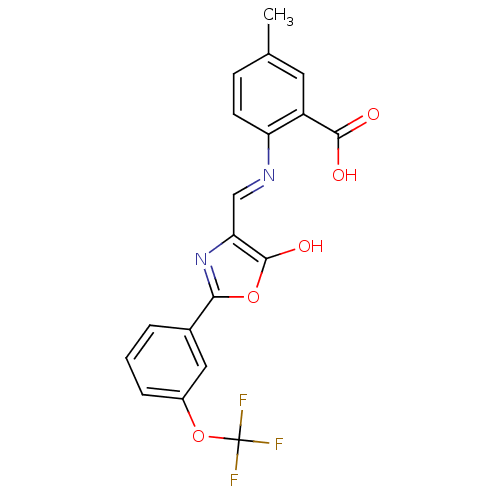
Affinity DataIC50: 130nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
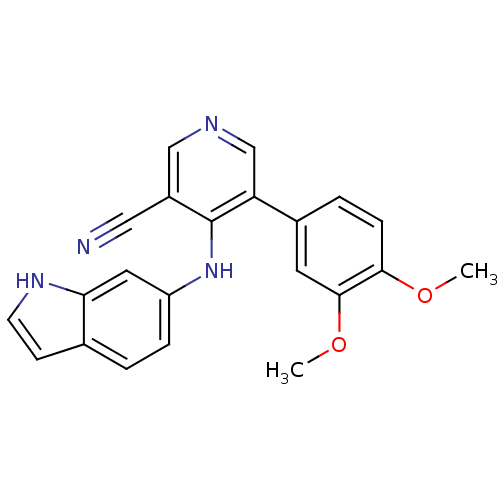
Affinity DataIC50: 160nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 174nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 270nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 470nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 830nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:In vitro inhibitory activity against acyl carrier protein synthase (AcpS) in Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+3nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:In vitro inhibitory activity against Holo-[acyl-carrier-protein] synthase was determined using Bacillus subtilis GST-Acp-HTRFassayMore data for this Ligand-Target Pair
TargetEnoyl-acyl carrier reductase ENR(Toxoplasma gondii)
University of Illinois at Chicago
Curated by ChEMBL
University of Illinois at Chicago
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Toxoplasma gondii enoyl reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMpH: 7.2 T: 2°CAssay Description:All IC50s were measured by using a modified IMAP protocol from Molecular Devices. The kinase reaction was carried out in a Corning Costar 384 well pl...More data for this Ligand-Target Pair