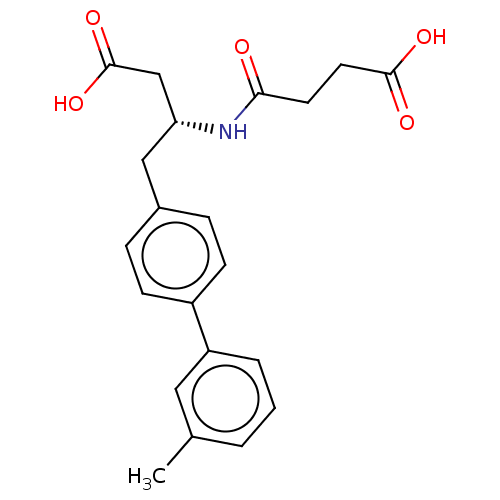
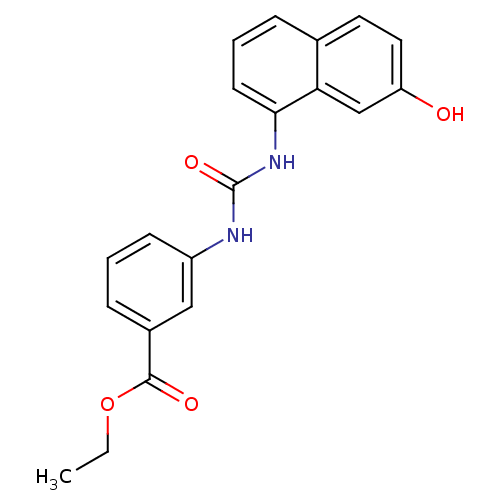
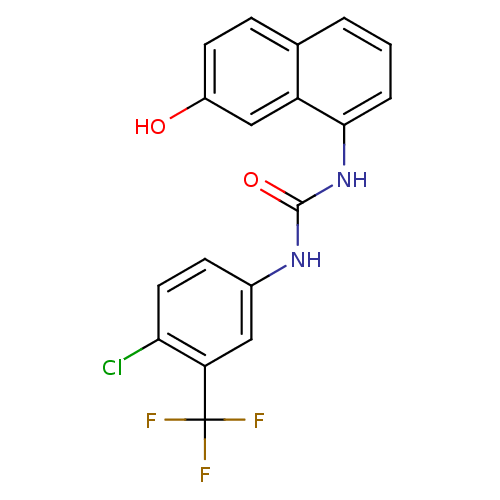
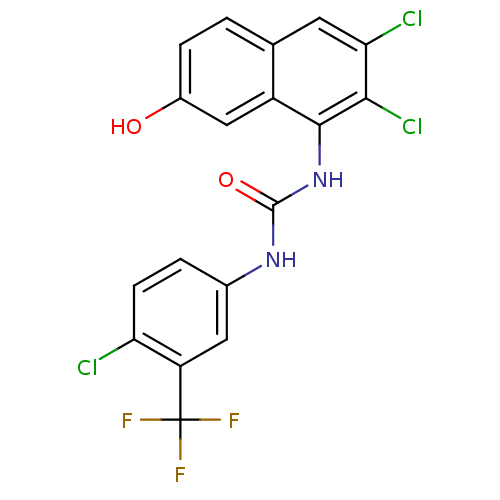
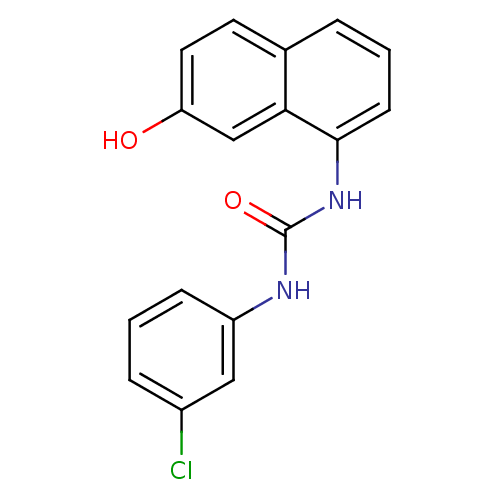
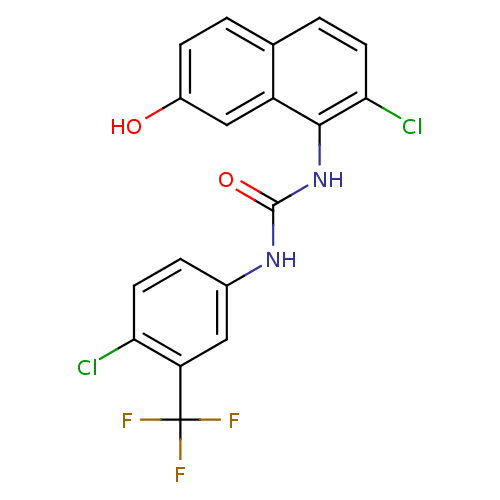
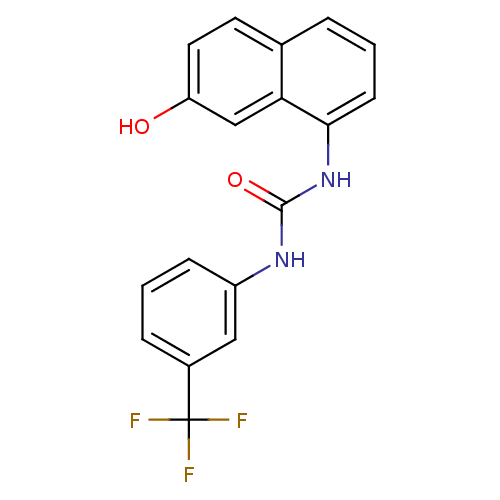
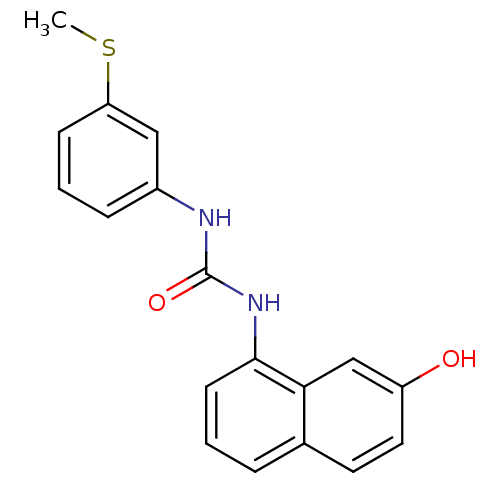
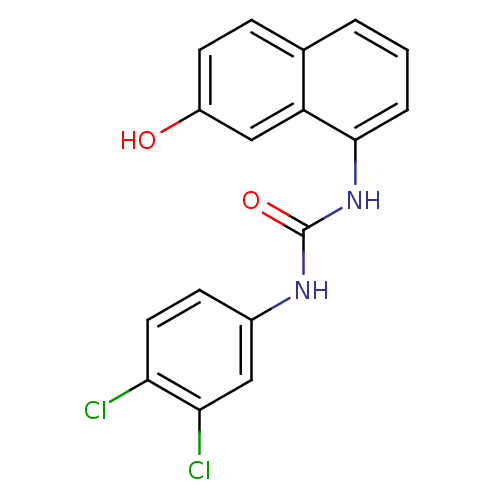
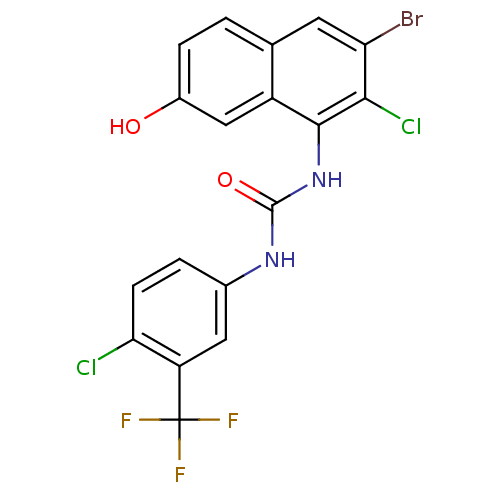
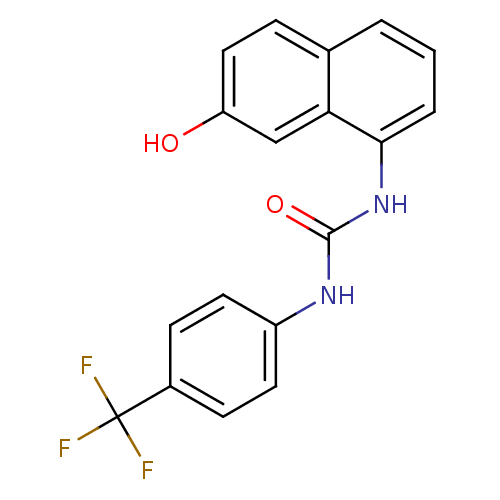
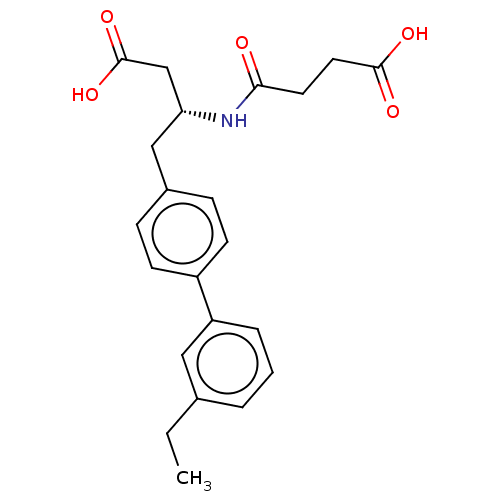
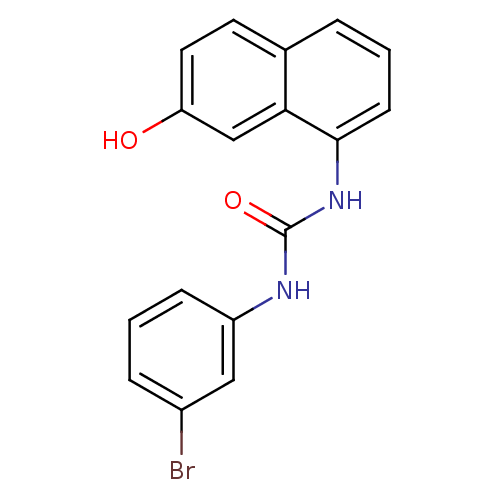
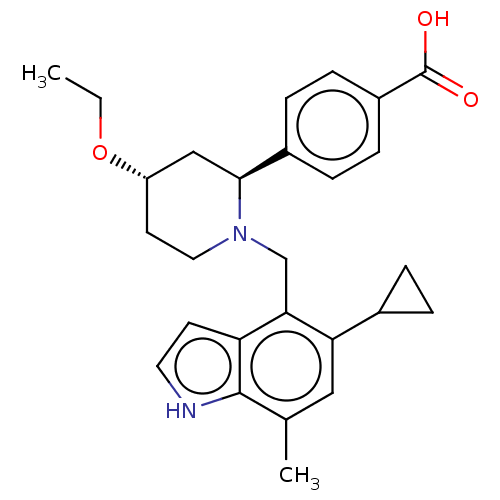
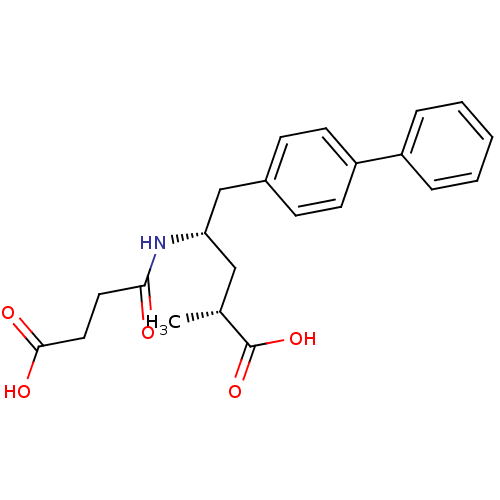
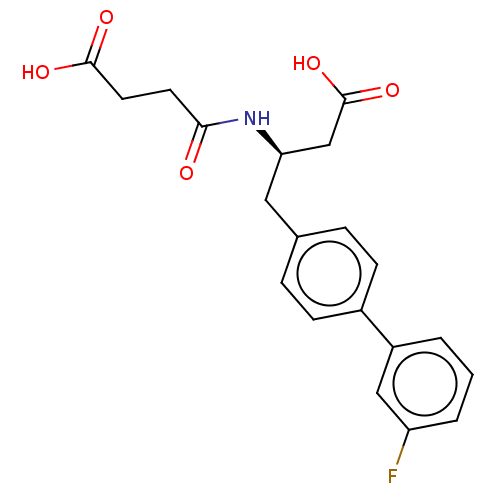
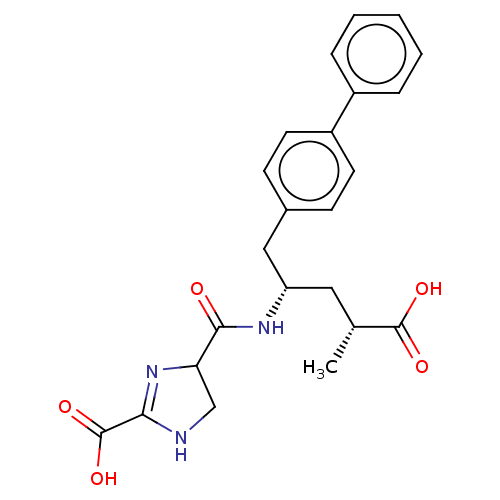
Affinity DataIC50: 0.0300nMAssay Description:The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMpH: 7.4 T: 2°CAssay Description:See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMpH: 7.4 T: 2°CAssay Description:See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4 T: 2°CAssay Description:See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.380nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.4 T: 2°CAssay Description:See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetComplement factor D(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Antagonist activity at human TRPV1 expressed in CHOluc9aeq cells assessed as inhibition of capsaicin-stimulated response by aequorin and CRE-lucifear...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.5nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
TargetComplement factor D(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of 2-((1E,3E,5E)-5-(1-(6-((((3S,5S)-1-((1-carbamoyl-1H-indol-3-yl)carbamoyl)-5-((3-chloro-2-fluorobenzyl)carbamoyl)-3-fluoropyrrolidin-3-y...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 5.40nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 5.70nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 5.90nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
TargetComplement factor B(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human serine protease factor B by TR-FRET based competition binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.3 T: 2°CAssay Description:Each well of the MBP-coated ScintiPlates held 100 ul of a solution containing 20 mM HEPES pH 7.3, 5 mM MnCl2 (WNK1 and WNK4) or 3 mM MnCl2 (WNK2 and ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:Antagonist activity at human TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin...More data for this Ligand-Target Pair
Affinity DataIC50: 6.90nMAssay Description:Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Rattus norvegicus (rat))
Bayer Yakuhin, Ltd
Curated by ChEMBL
Bayer Yakuhin, Ltd
Curated by ChEMBL
Affinity DataIC50: 7.10nMAssay Description:Antagonist activity at rat TRPV1 expressed in CHO cells co-expressing aequorin and CRE-luciferase reporter gene assessed as inhibition of capsaicin-i...More data for this Ligand-Target Pair
Affinity DataIC50: 7.30nMAssay Description:The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ...More data for this Ligand-Target Pair

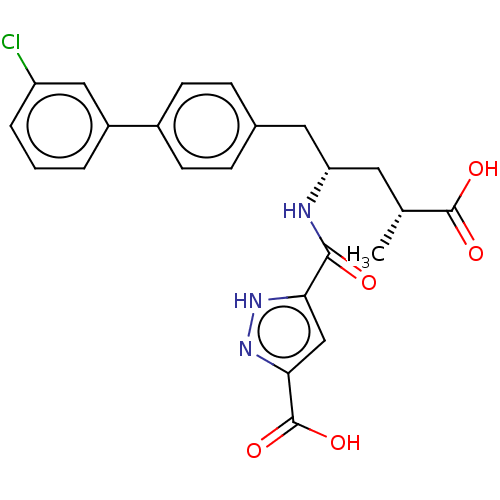
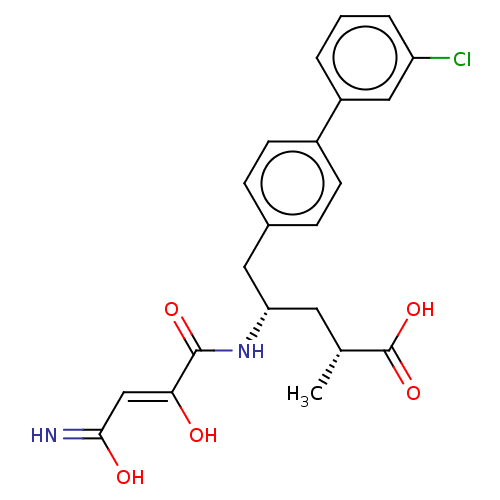
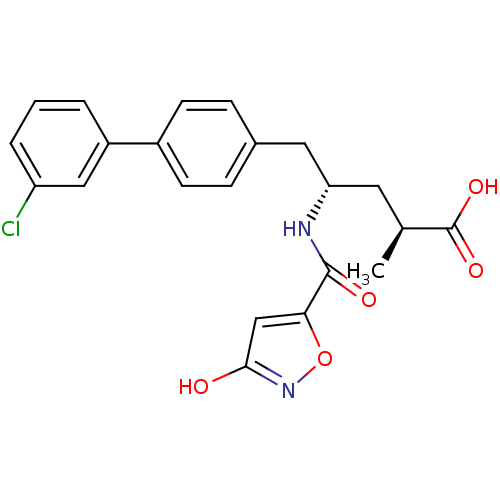
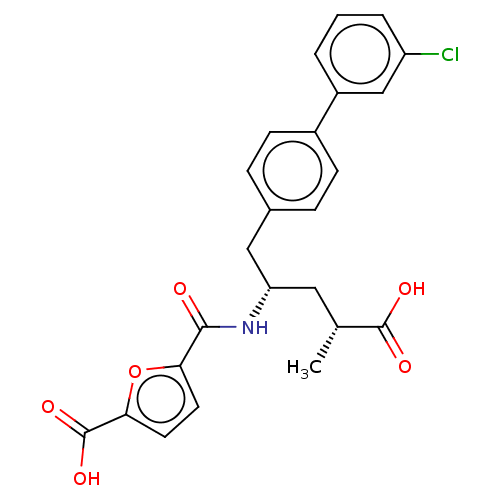
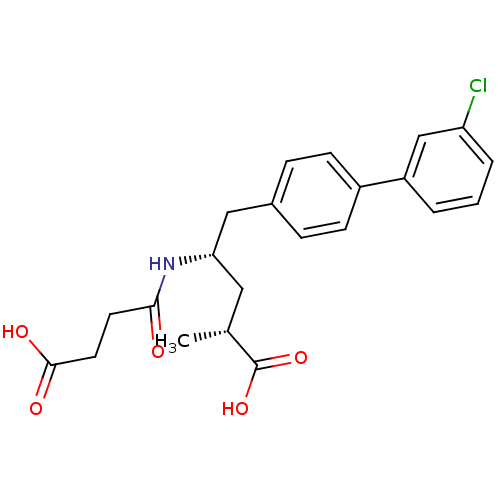
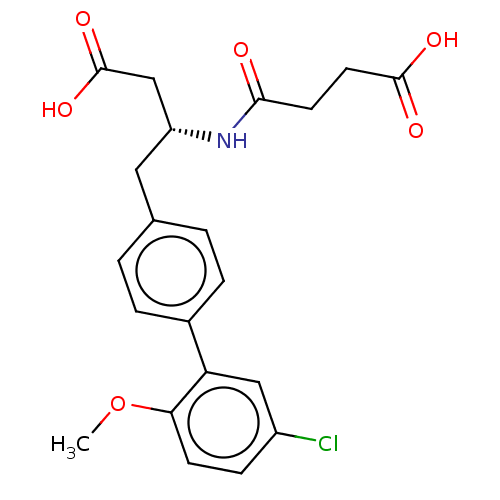
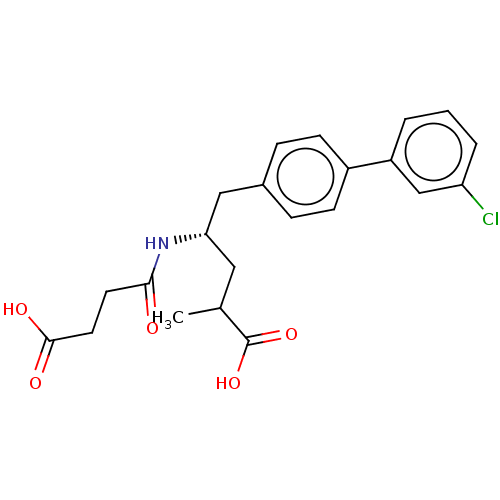
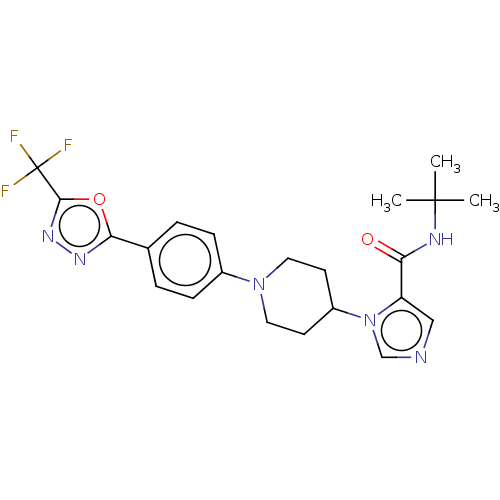

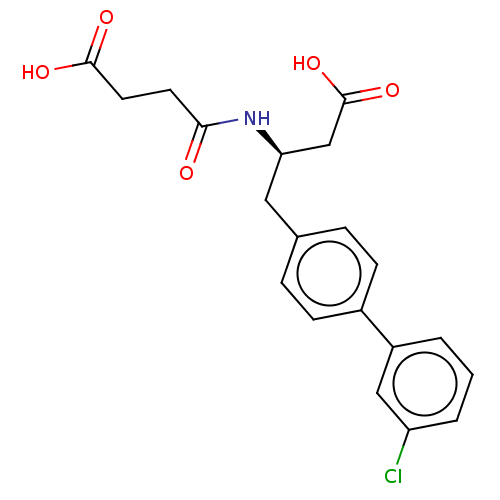
 3D Structure (crystal)
3D Structure (crystal)