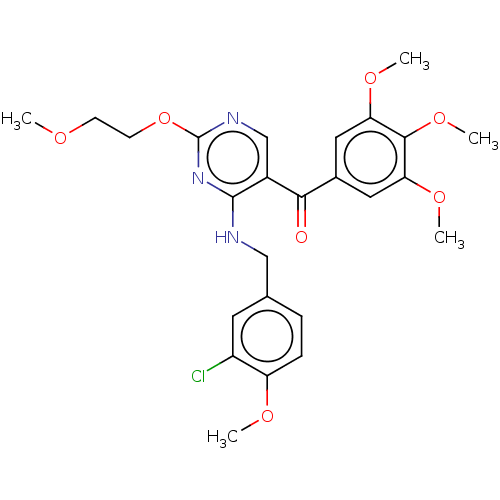
Affinity DataKi: 9.10nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 9.80nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Binding Affinity towards Adenosine A2A receptor expressed in HEK-293 cells versus [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 71nMAssay Description:Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:Binding affinity towards adenosine A3 receptor expressed in HEK-293 cells versus [125I]-AB-MECAMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding Affinity towards Adenosine A1 receptor expressed in CHO-K1 cells versus [3H]-CCPAMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding Affinity for adenosine A2A receptor expressed in HEK-293 cells compared to [3H]-CGS-21,680More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 0.0530nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 0.130nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 0.380nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 0.660nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 0.740nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of dog lungs PDE5 using [3H]cGMP as substrate after 30 mins by scintillation counting analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibitory activity against cyclic AMP production in rat Adenosine A2A receptor assayMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of PDE5 (unknown origin)More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of dog lungs PDE5 using [3H]cGMP as substrate after 30 mins by scintillation counting analysisMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1.5nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of PDE5 (unknown origin)More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of canine lung PDE5 using [3H]cGMP substrate by radiolabeled nucleotide methodMore data for this Ligand-Target Pair
TargetALK tyrosine kinase receptor(Homo sapiens (Human))
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba...More data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Canis lupus familiaris)
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Mitsubishi Tanabe Pharma Corporation
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of dog lungs PDE5 using [3H]cGMP as substrate after 30 mins by scintillation counting analysisMore data for this Ligand-Target Pair

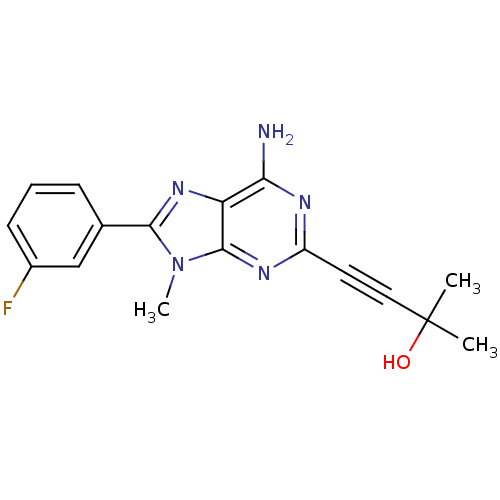
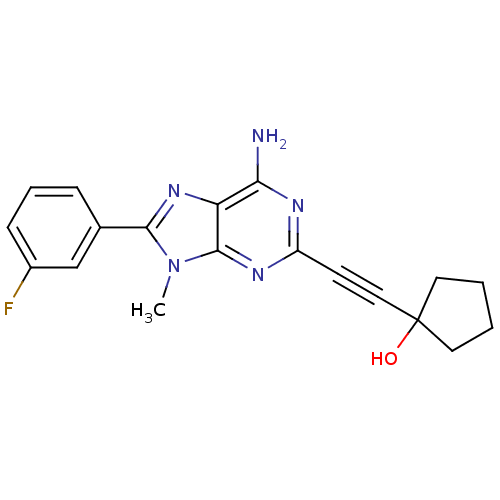
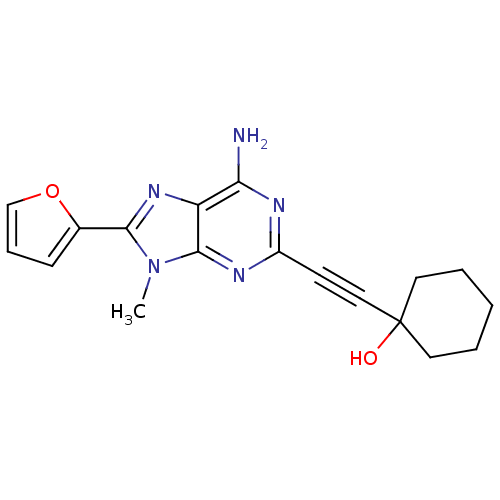


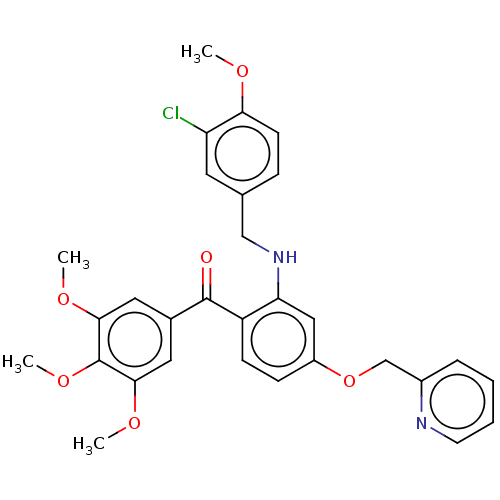
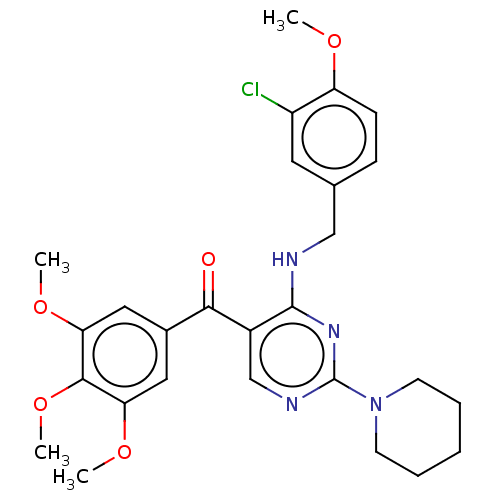
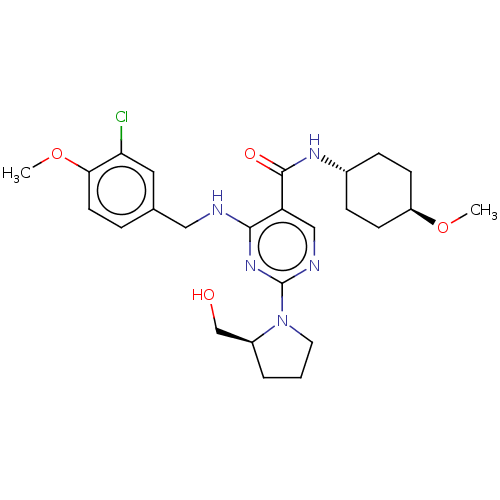


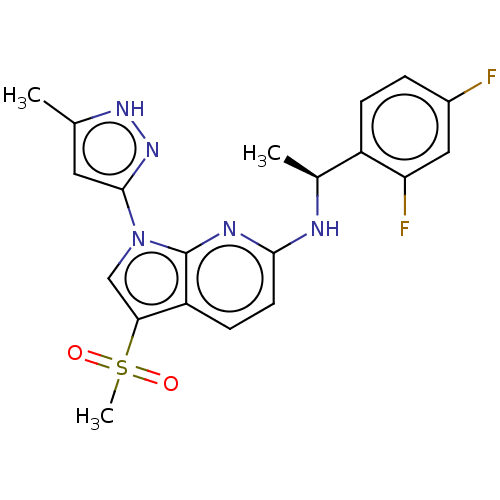

 3D Structure (crystal)
3D Structure (crystal)