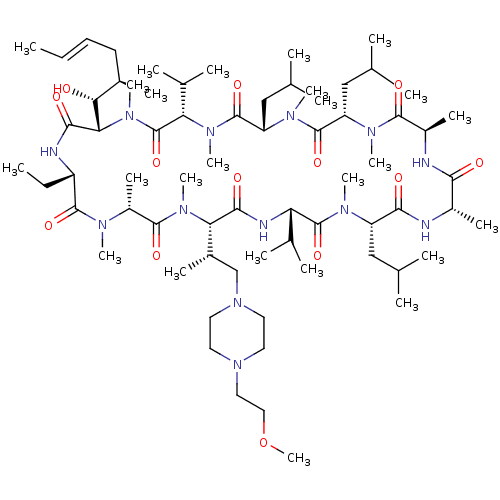
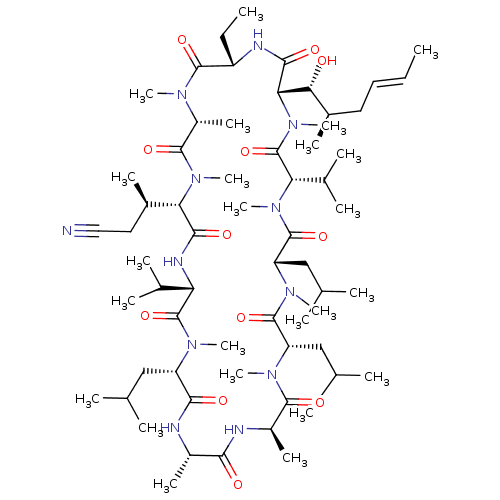
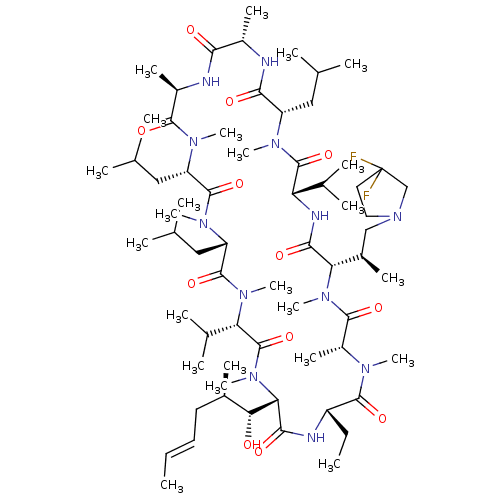
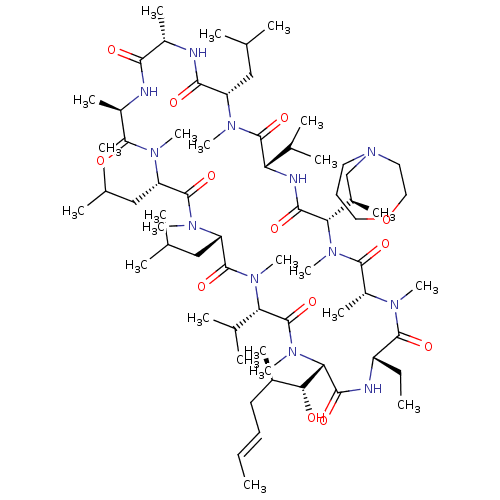
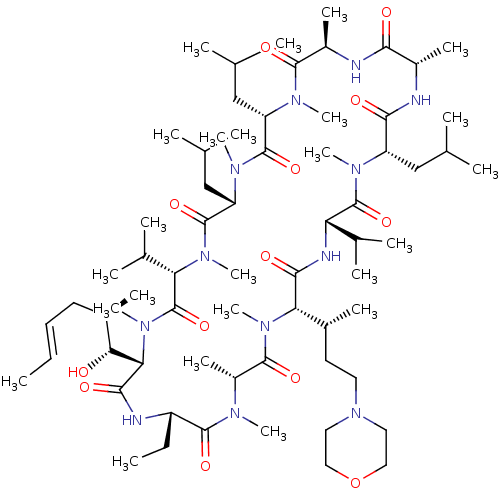
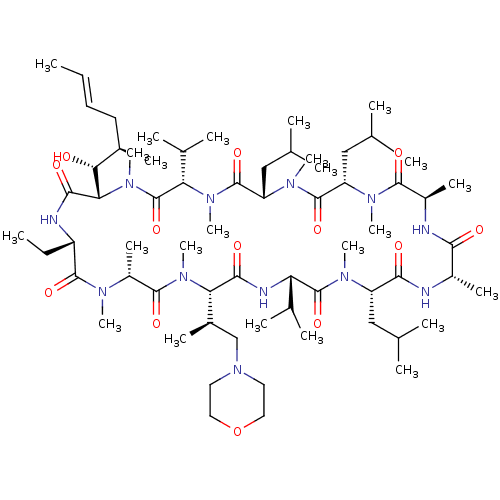
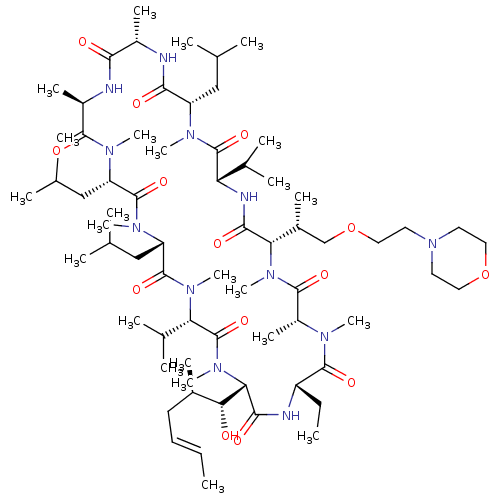
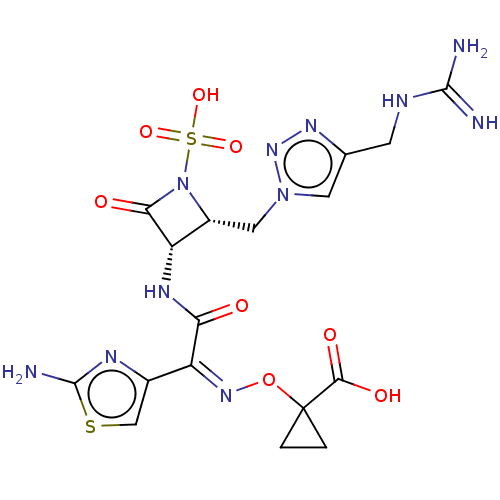
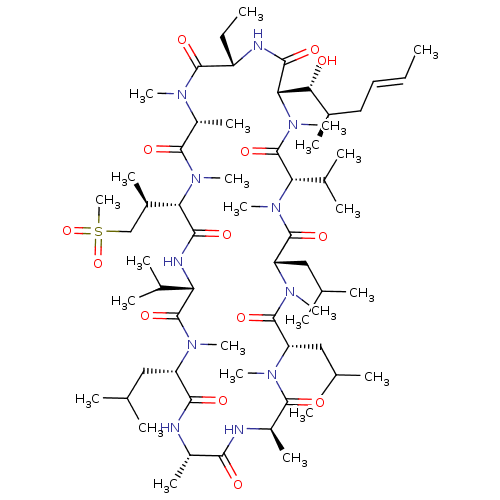
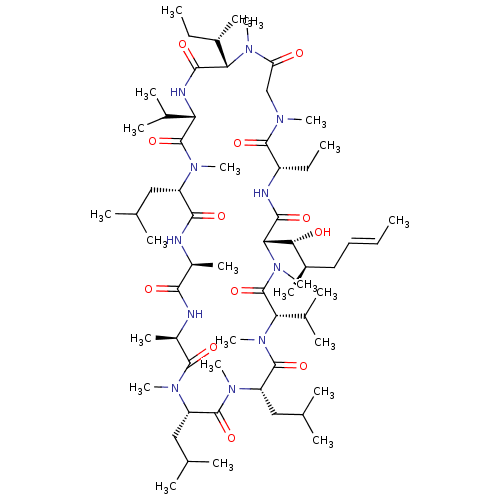
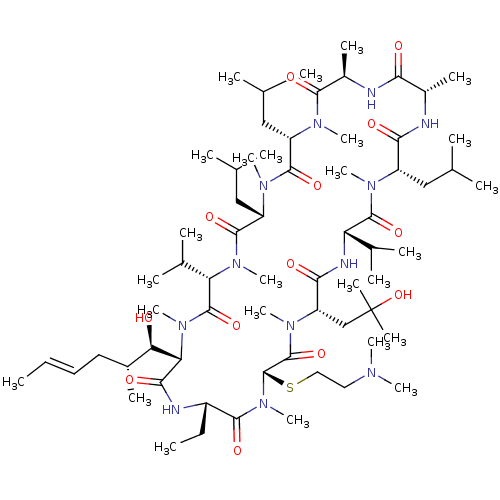
TargetBile salt export pump(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 590nMAssay Description:Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B3(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 880nMAssay Description:Inhibition of OATP1B3 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in HEK293 cells using [3H]estradiol-17beta-glucuronide substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetBile salt export pump(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of BSEP (unknown origin) expressed in HEK293 cells using [3H]taurocholic acid substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.10E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of MDR1 (unknown origin) expressed in MDA T0.3 cells using rhodamine 123 substrateMore data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 1B1(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6.40E+3nMAssay Description:Inhibition of OATP1B1 (unknown origin) expressed in CHO cells using 8-fluorescein-cAMP substrate by fluorescent photometryMore data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrateMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of BCRP (unknown origin) expressed in T8 cells using Bodipy FL-prazosin substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2CJ (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C8(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 2(Homo sapiens (Human))
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Novartis Institutes for Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.40E+5nMAssay Description:Inhibition of human MRP2 expressed in Sf9 cells inside out vesicles using CDCF substrateMore data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.90E+5nMAssay Description:Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 8.90E+5nMAssay Description:Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 1.34E+6nMAssay Description:Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA...More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-2/beta-2/gamma-2(Homo sapiens (Human))
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Novartis Institutes for BioMedical Research
Curated by ChEMBL
Affinity DataIC50: 2.40E+6nMAssay Description:Inhibition of recombinant human GABA A alpha2beta2gamma2 receptor expressed in CHO-K1 cells incubated at room temperature for 15 mins before the GABA...More data for this Ligand-Target Pair
Affinity DataKd: 2.05nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 1.45nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase F, mitochondrial(Homo sapiens (Human))
Novartis AG
US Patent
Novartis AG
US Patent
Affinity DataKd: 1.30nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 1.70nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 0.800nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase F, mitochondrial(Homo sapiens (Human))
Novartis AG
US Patent
Novartis AG
US Patent
Affinity DataKd: 0.800nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 12.7nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 5.20nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase F, mitochondrial(Homo sapiens (Human))
Novartis AG
US Patent
Novartis AG
US Patent
Affinity DataKd: 5nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 1.5nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 0.600nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
TargetPeptidyl-prolyl cis-trans isomerase F, mitochondrial(Homo sapiens (Human))
Novartis AG
US Patent
Novartis AG
US Patent
Affinity DataKd: 0.600nMpH: 7.4Assay Description:Binding of inhibitors to expressed cyclophilins was determined using surface plasmon resonance (SPR) experiments. Briefly, avi-tagged cyclophilin pro...More data for this Ligand-Target Pair
Affinity DataKd: 2.20nMAssay Description:Binding affinity to human cyclophilin A by surface plasmon resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: 7.5nMAssay Description:Binding affinity to human cyclophilin A by surface plasmon resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: 7.40nMAssay Description:Binding affinity to human cyclophilin B by surface plasmon resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: 1nMAssay Description:Binding affinity to human cyclophilin B by surface plasmon resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: 2.90nMAssay Description:Binding affinity to human cyclophilin B by surface plasmon resonance methodMore data for this Ligand-Target Pair
Affinity DataKd: 1.20nMAssay Description:Binding affinity to human cyclophilin B by surface plasmon resonance methodMore data for this Ligand-Target Pair