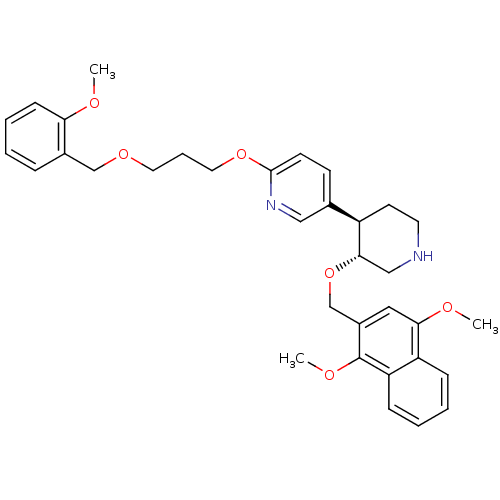
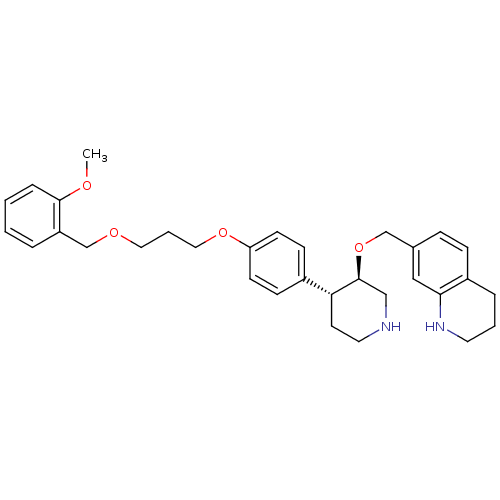
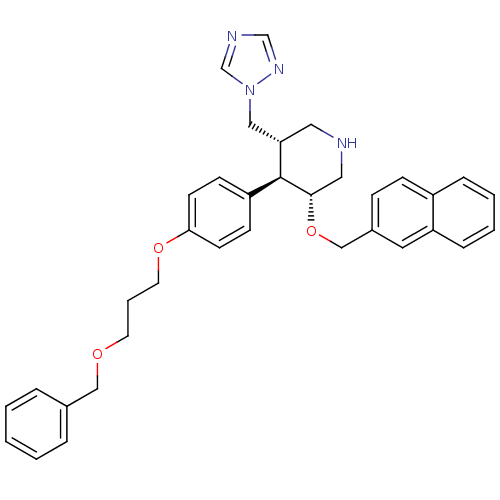
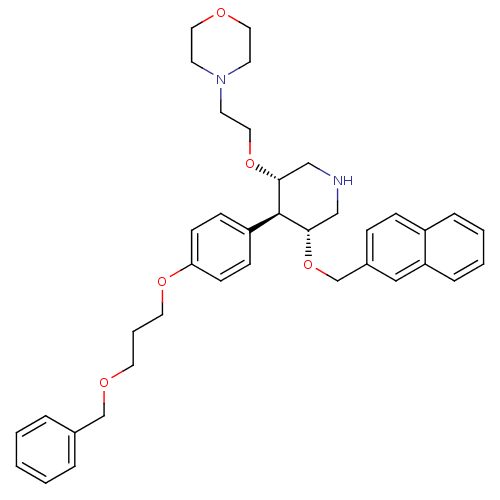
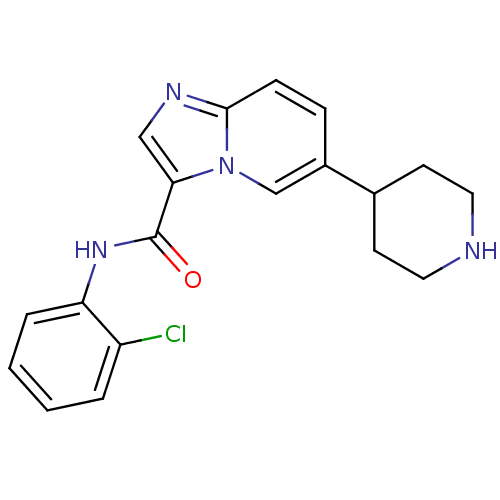
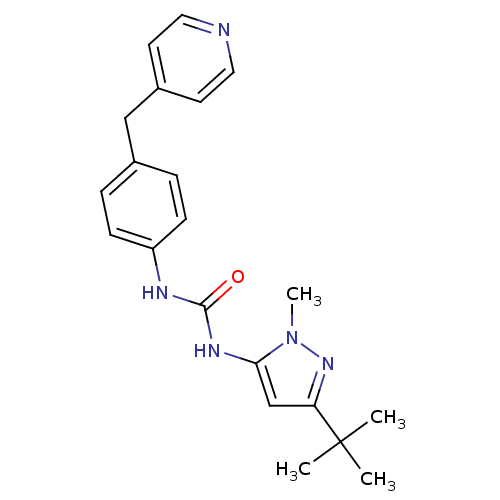
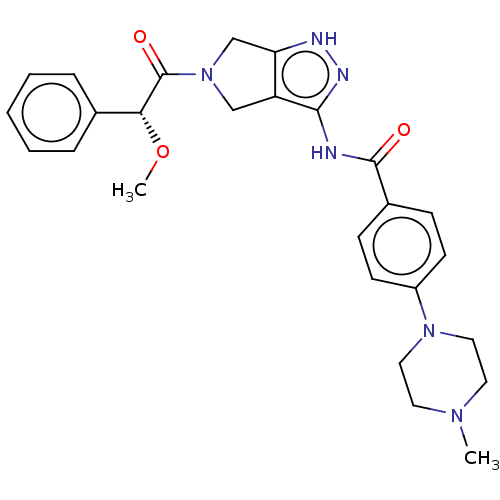
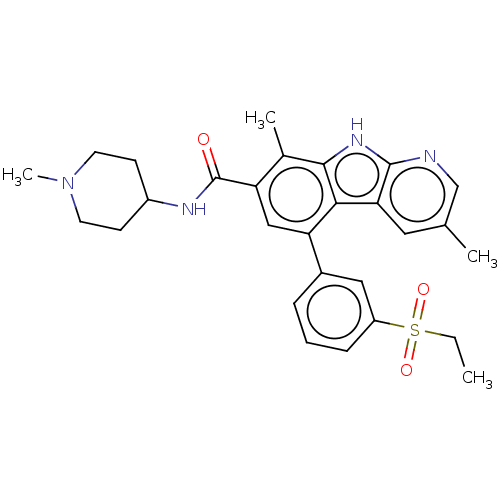
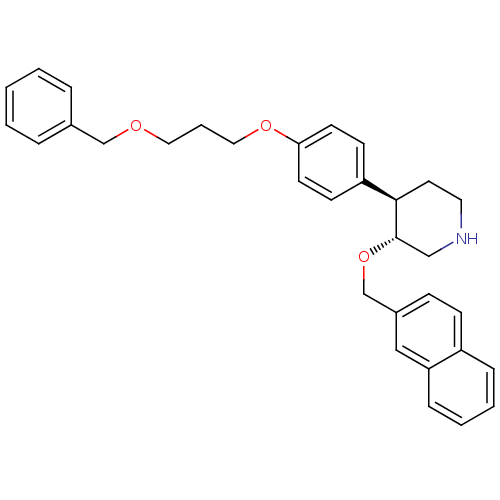
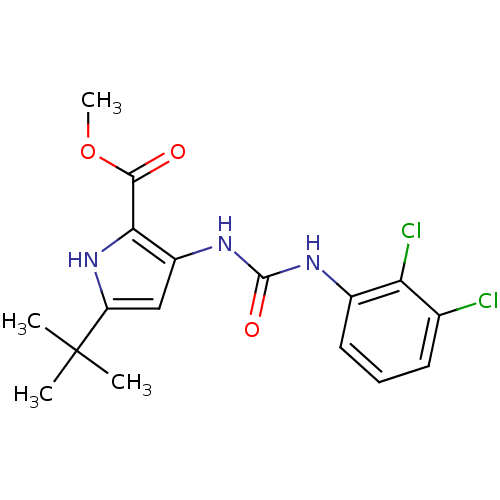
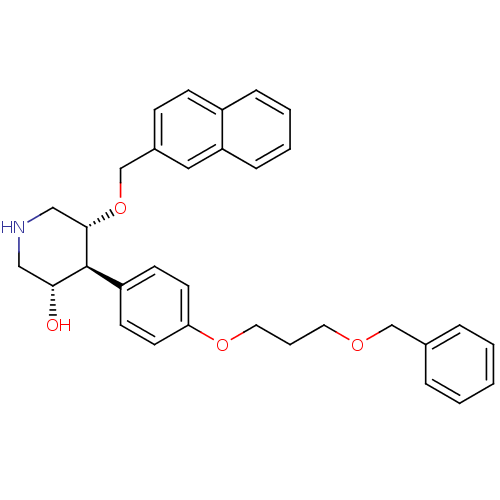
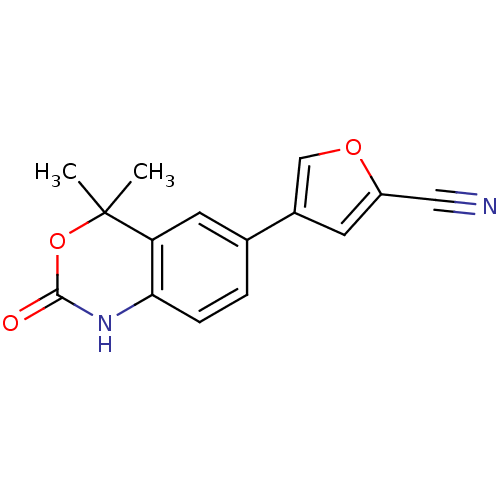
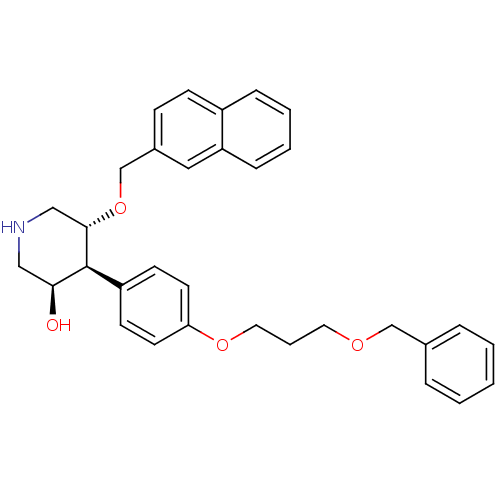
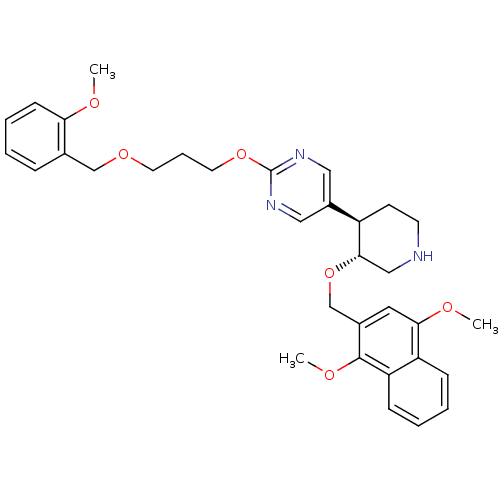
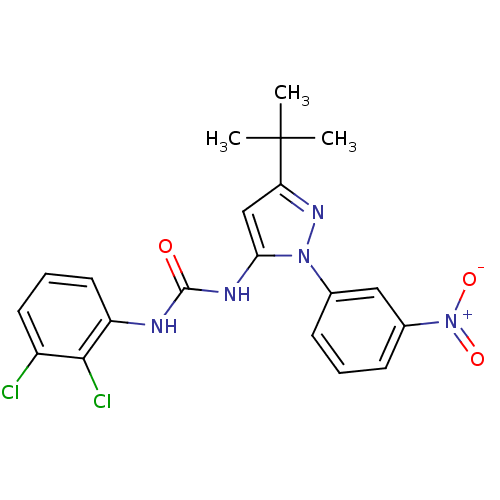
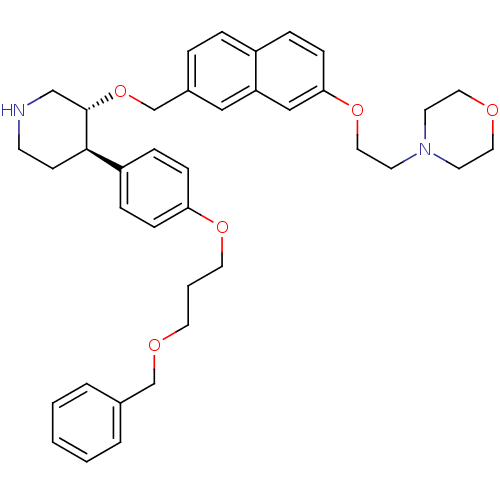
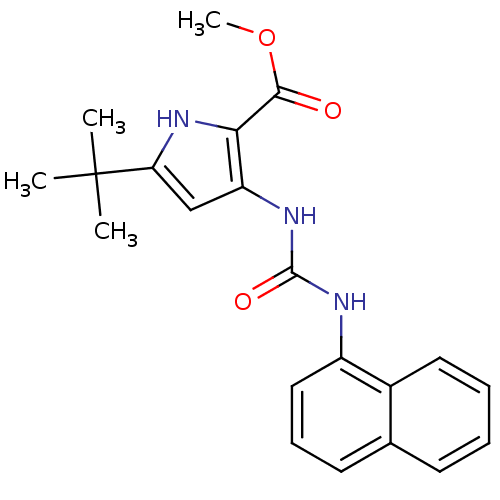
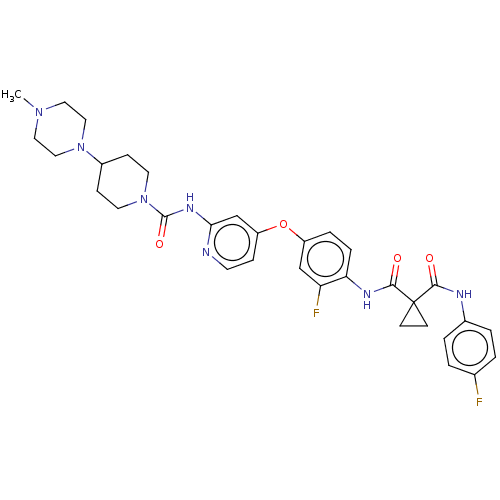
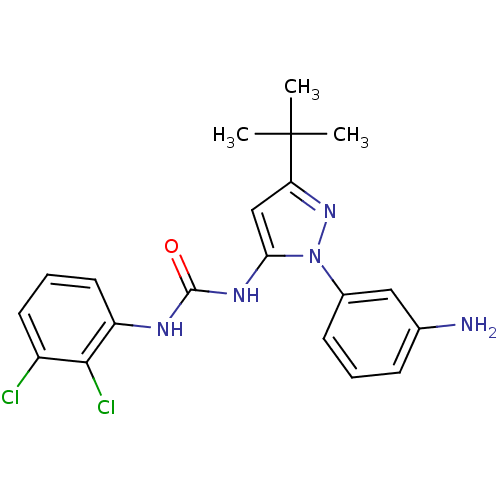
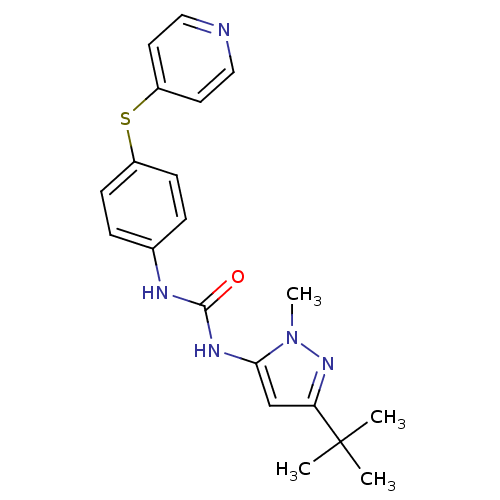
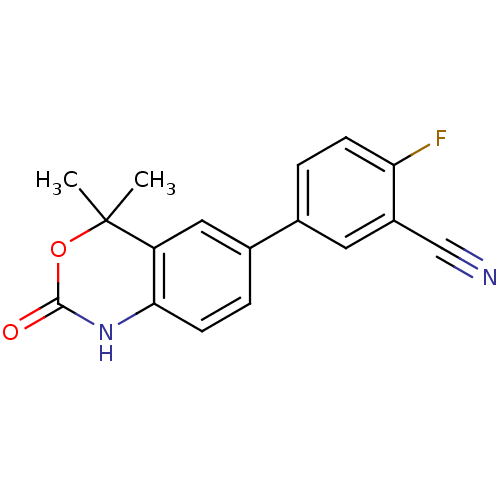
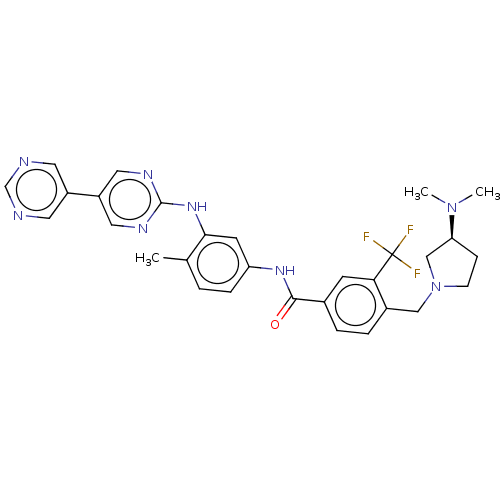
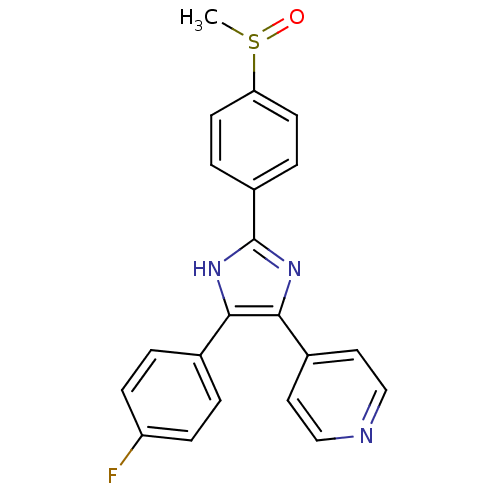
Affinity DataIC50: 0.0250nMAssay Description:In vitro inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:In vitro inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0870nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Inhibitory activity against human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:In vitro inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 expressed in E. coli.More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibitory activity against human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of human epidermal growth factor receptor-2More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coliMore data for this Ligand-Target Pair
TargetRAF proto-oncogene serine/threonine-protein kinase [305-648](Homo sapiens (Human))
Bayer Pharmaceuticals Corporation
Bayer Pharmaceuticals Corporation
Affinity DataIC50: 6nMpH: 8.2 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Raf kinases and substrate MEK-1 in the presence of 1-10 uM ATP/ [gamma-32P] AT...More data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:In vitro inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 9nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of mitogen-activated protein kinase p38 alpha 2More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against human plasma reninMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against purified recombinant human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against human plasma reninMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coliMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of mitogen-activated protein kinase p38 alpha 2More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2More data for this Ligand-Target Pair
Affinity DataIC50: 15nMpH: 7.5 T: 2°CAssay Description:Kinase assays were performed using the europium/APC detection format (LANCE, Perkin Elmer). HTRF is based on the proximity of europium cryptate (dono...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human progesterone receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Refer to Reaction Biology Corps.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of mitogen-activated protein kinase p38 alpha 2More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 3(Mus musculus (mouse))
Bayer Pharmaceuticals Corporation
Bayer Pharmaceuticals Corporation
Affinity DataIC50: 20nMpH: 7.5 T: 2°CAssay Description:Kinase assays were performed using the europium/APC detection format (LANCE, Perkin Elmer). HTRF is based on the proximity of europium cryptate (dono...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:In vitro inhibition of mitogen-activated protein kinase p38 alpha 2 derived from E. coliMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2 expressed in E. coli.More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Bayer Research Center
Curated by ChEMBL
Bayer Research Center
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against Mitogen-activated protein kinase p38 alpha 2More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)