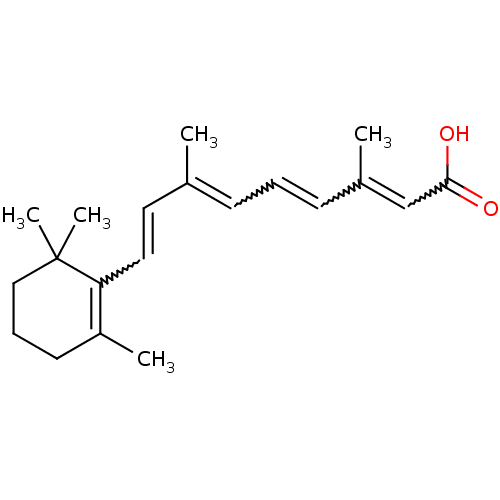
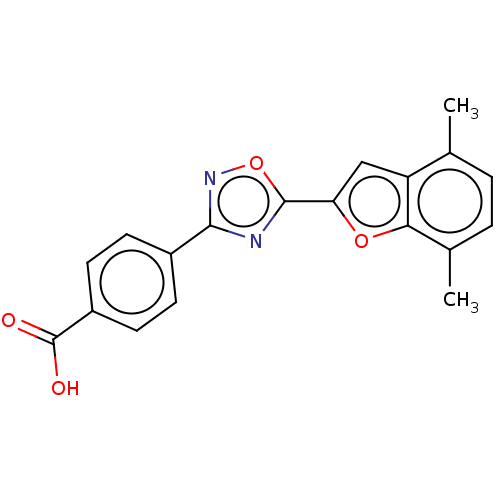
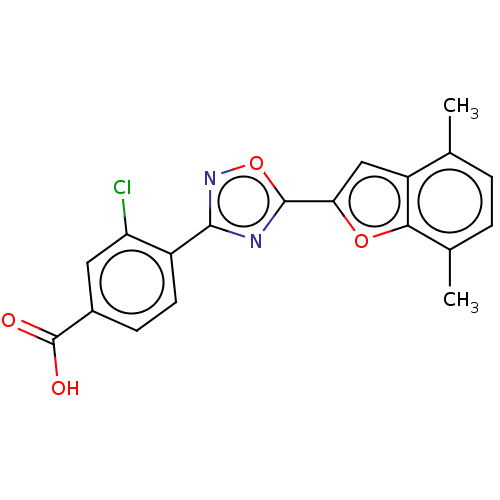
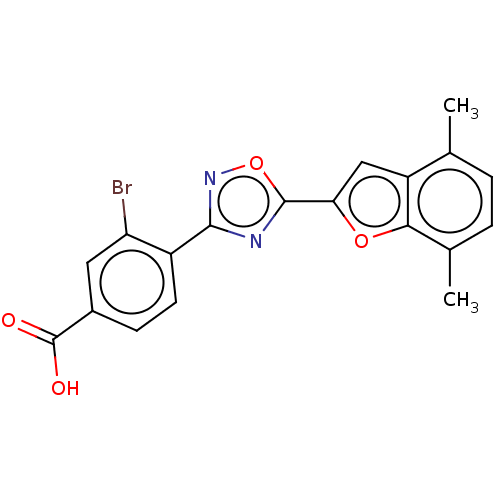
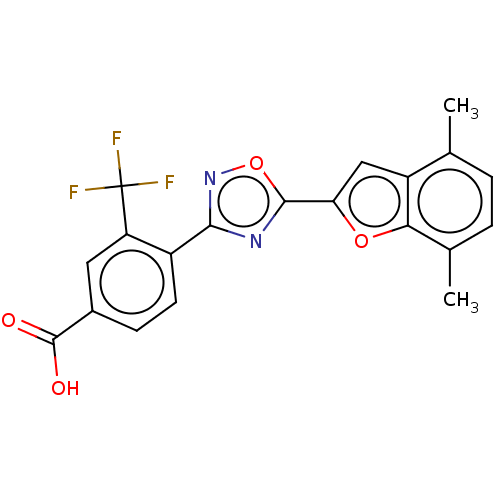
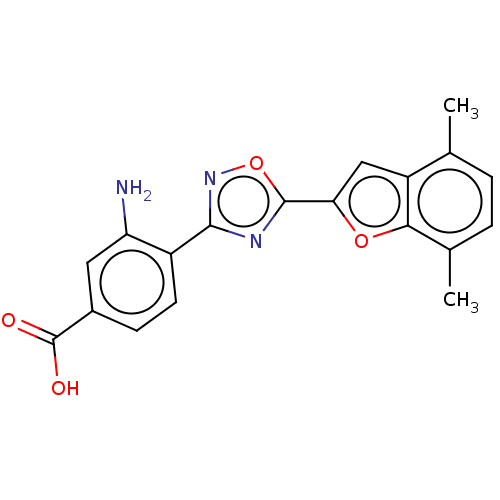
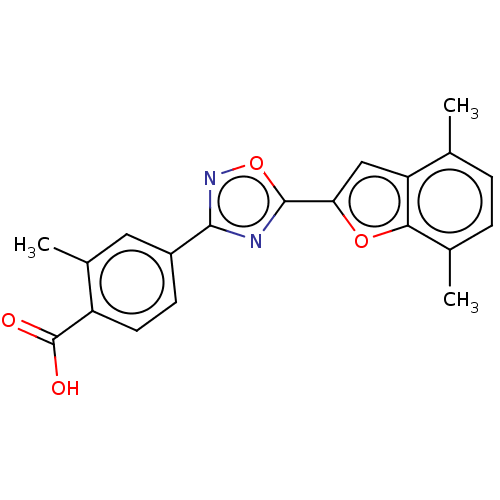
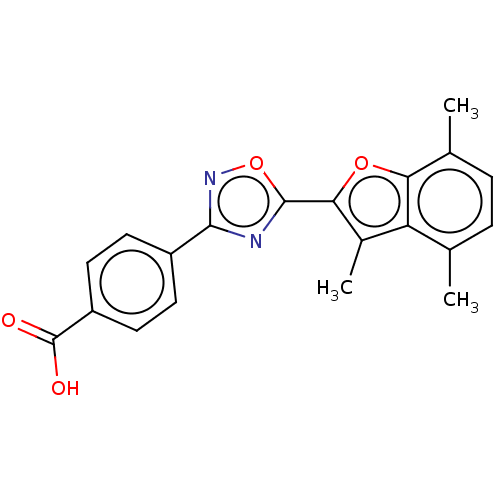
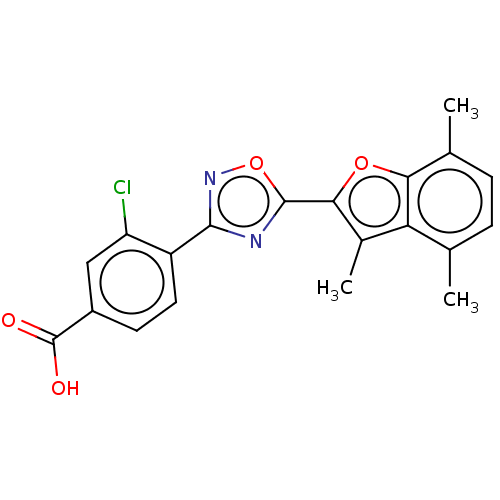
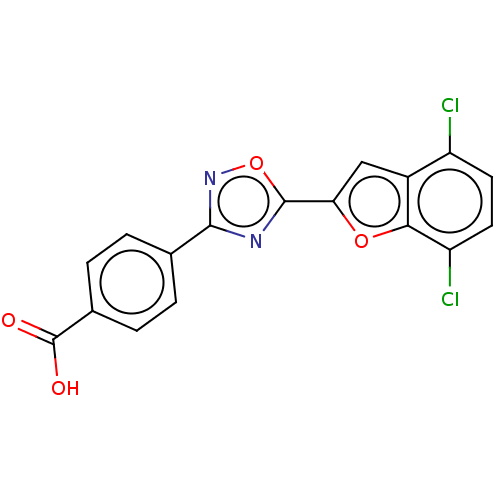
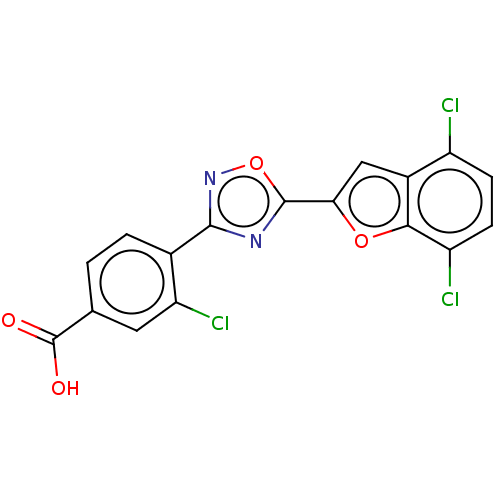
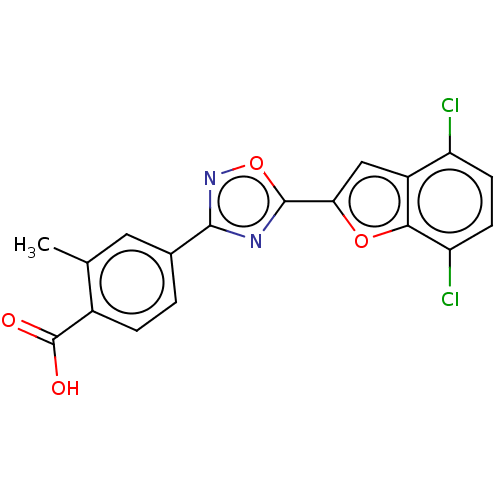
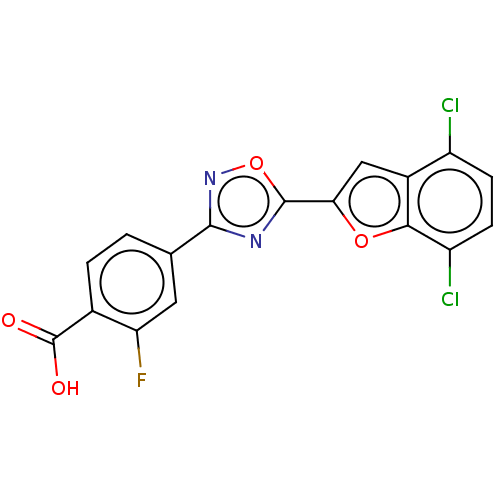
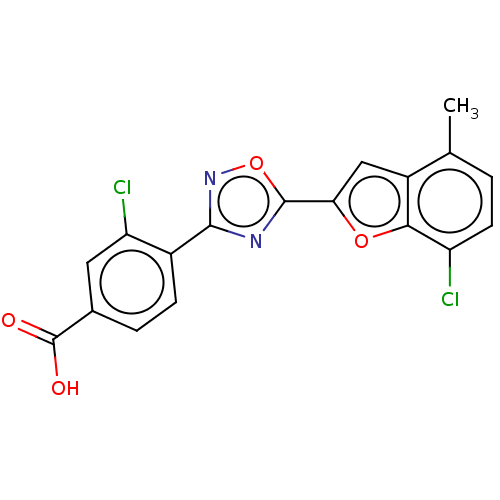
Affinity DataEC50: 1.88nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 1.20nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 0.900nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 1.94nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 11.4nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 136nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 29nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 36nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 10nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 55nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 89nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 2.20nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 8.40nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 1.90nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 6.80nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 4.20nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 32nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 2.90nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 25nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 0.530nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 4.30nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 26nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 14nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 67nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 140nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 110nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 2.90nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 2.70nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 18nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair
Affinity DataEC50: 7.40nMAssay Description:Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e...More data for this Ligand-Target Pair