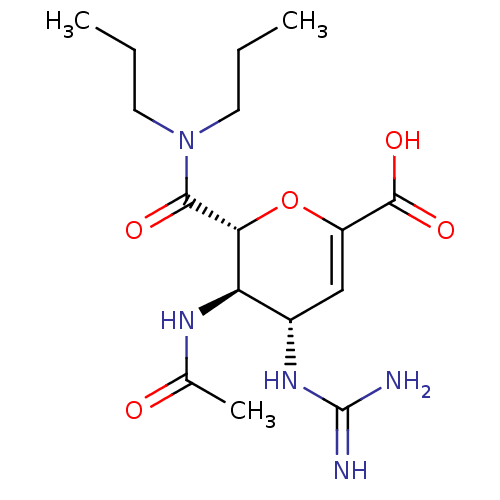
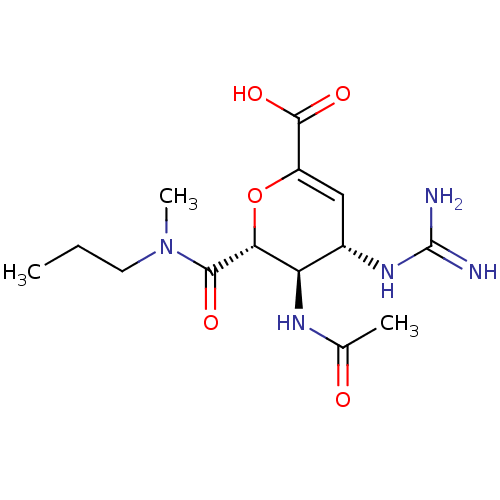
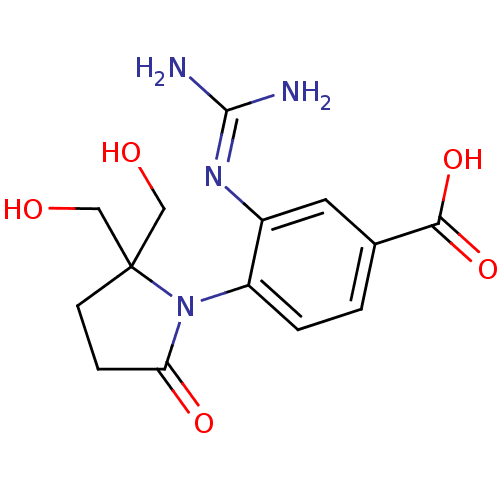
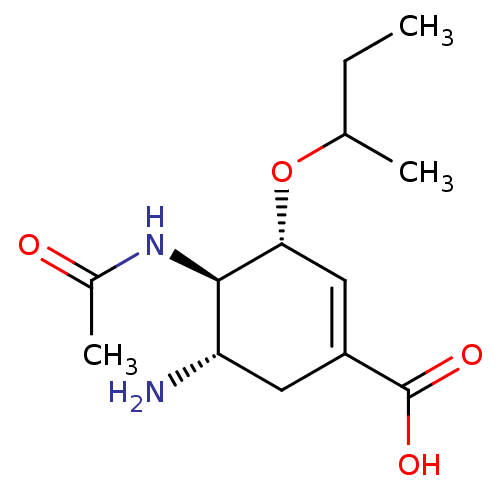
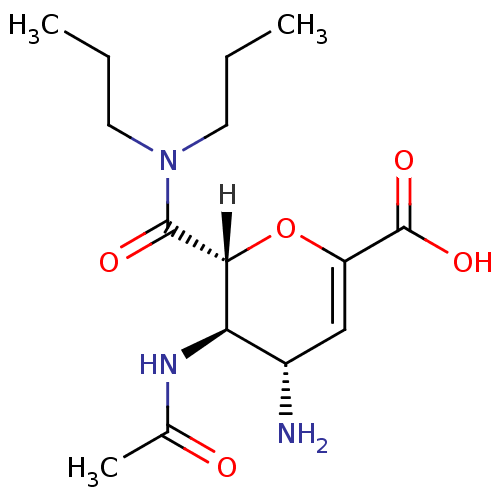
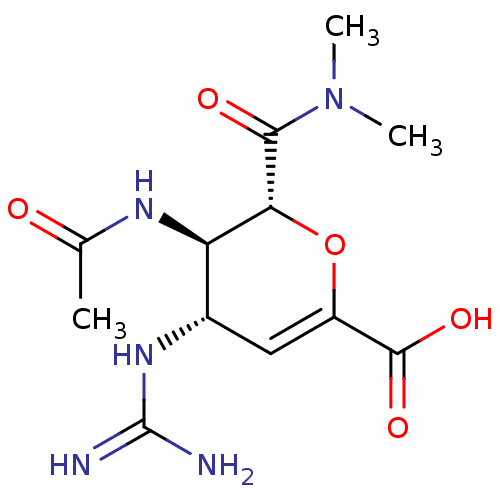
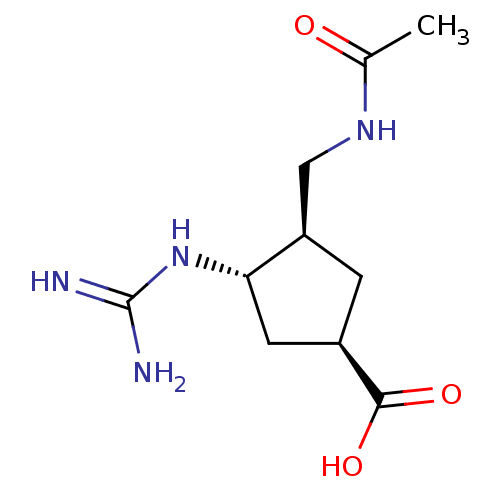
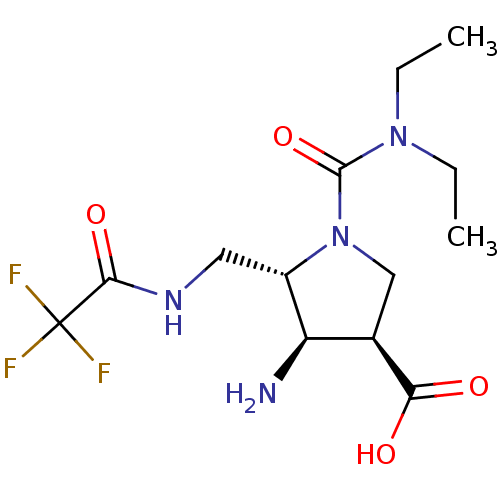
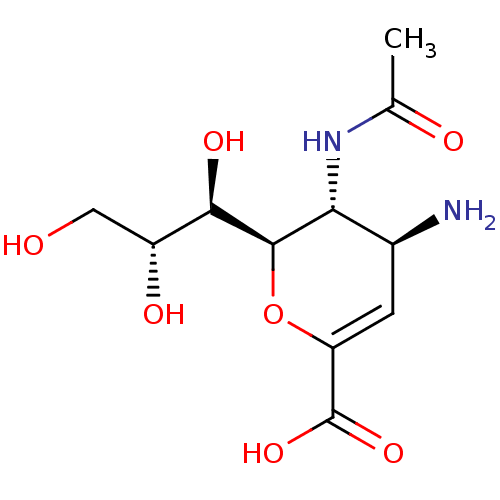
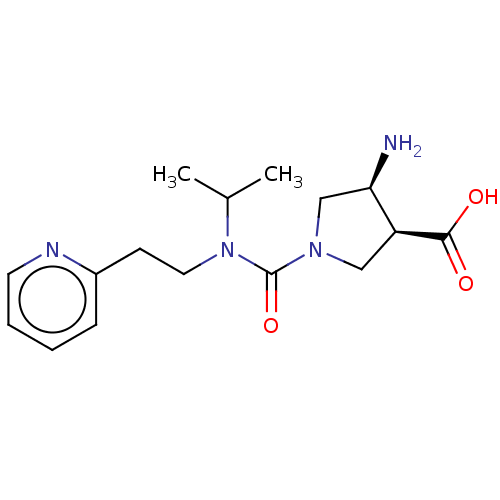
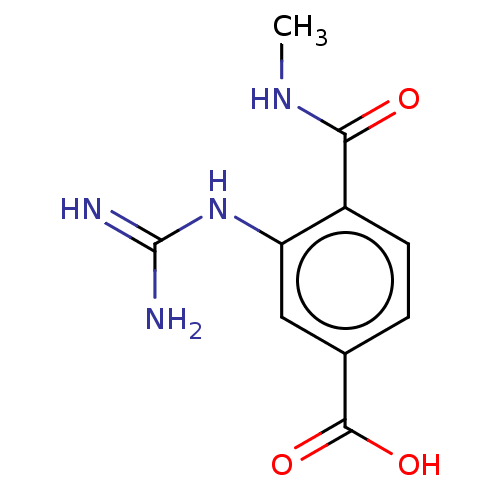
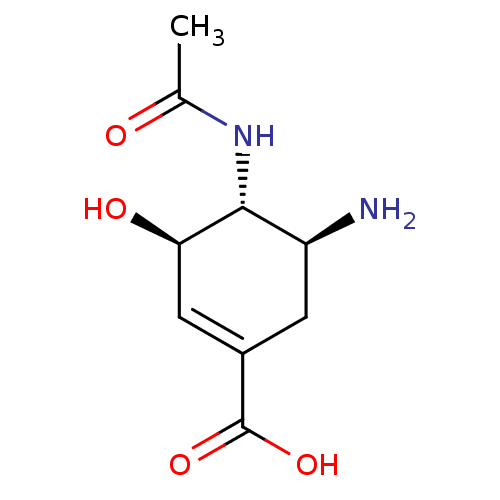
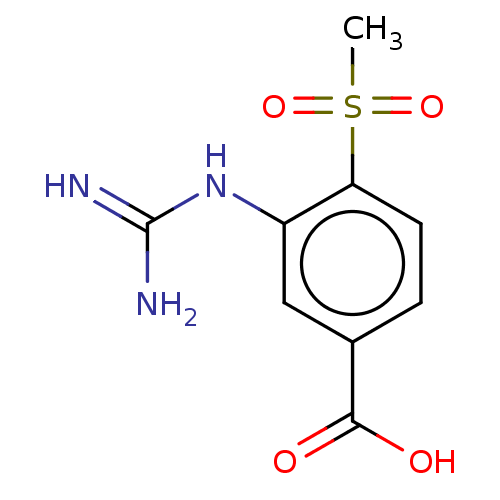
Affinity DataKi: 1.90E+5nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
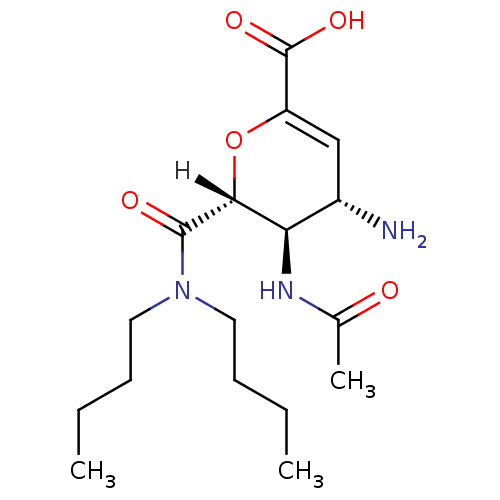
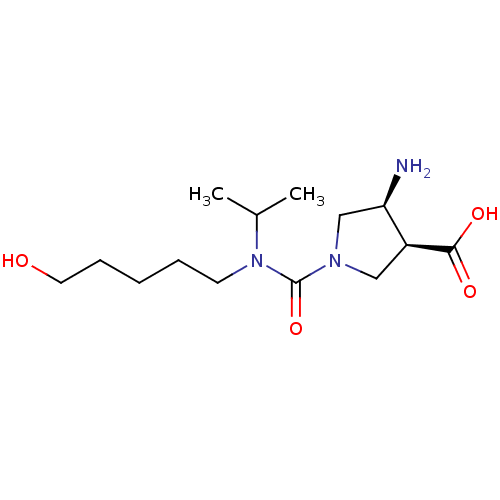
Affinity DataKi: 1.60E+6nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
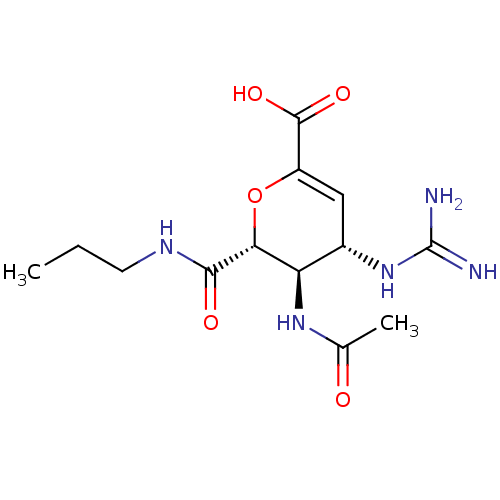
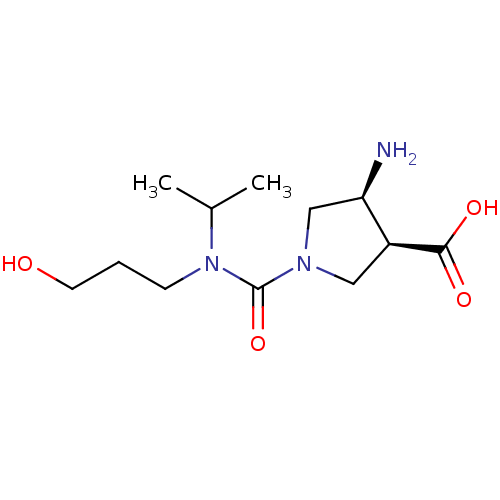
Affinity DataKi: 7.69E+6nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
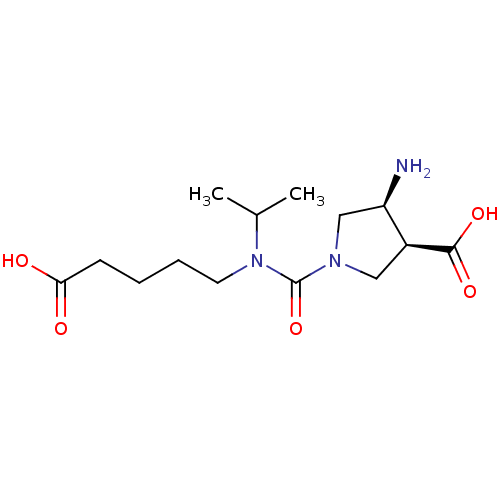
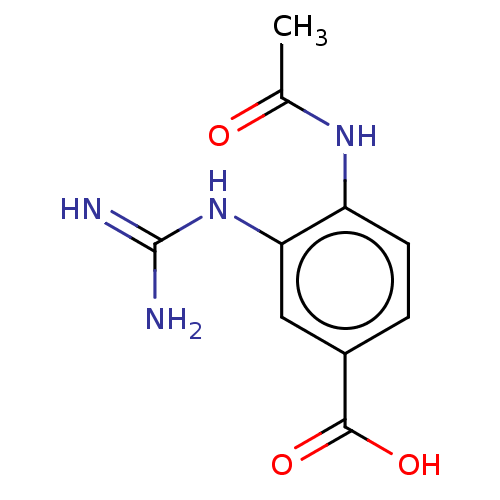
Affinity DataKi: 2.51E+7nMAssay Description:Inhibitory constant was measured on cytidine/deoxycytidine deaminaseMore data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 820nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 9.00E+3nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase subunit M2(Homo sapiens (Human))
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibitory activity against CDP reductase.More data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+4nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+5nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+6nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+7nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.39E+7nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+8nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.30E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 8.60E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+9nMAssay Description:Inhibition of influenza virus neuraminidaseMore data for this Ligand-Target Pair

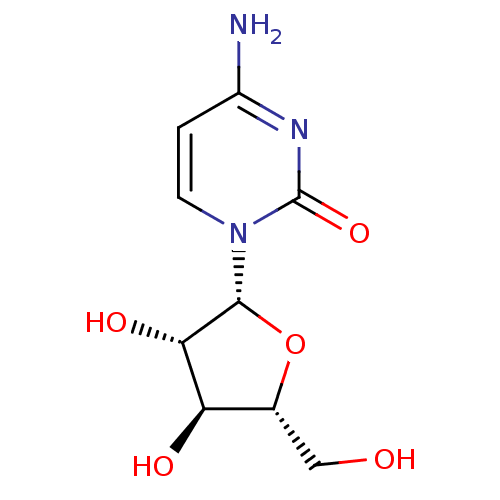
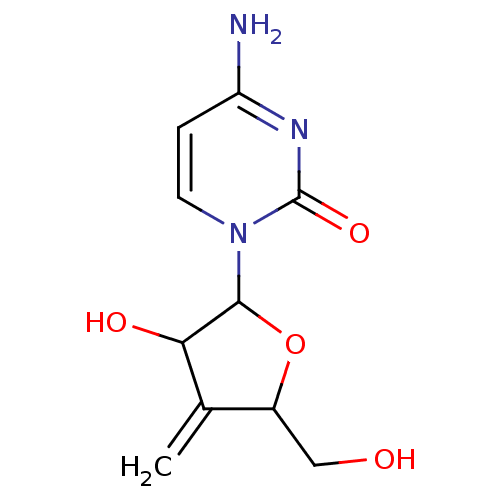
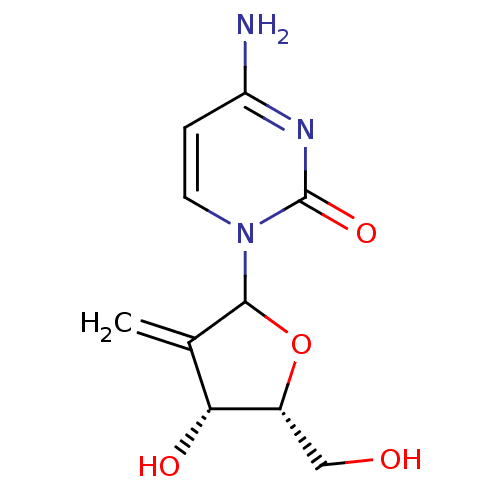
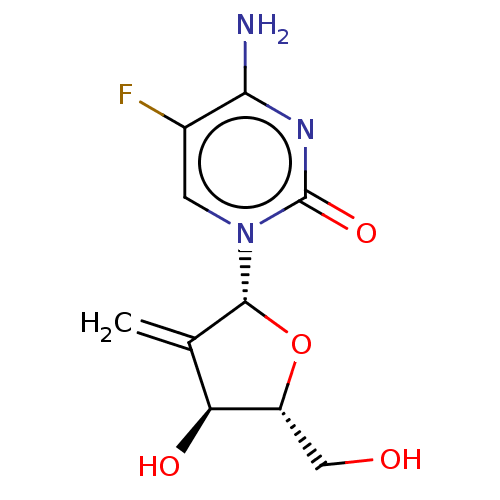
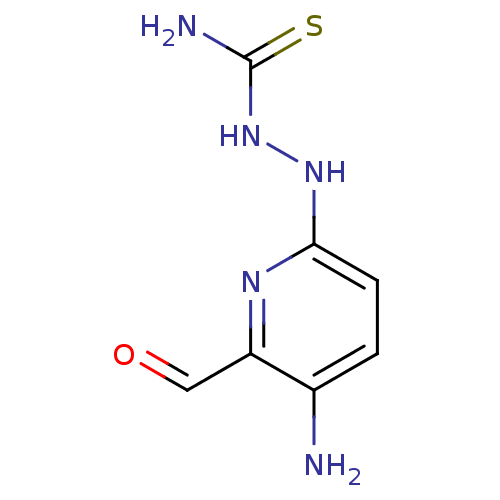
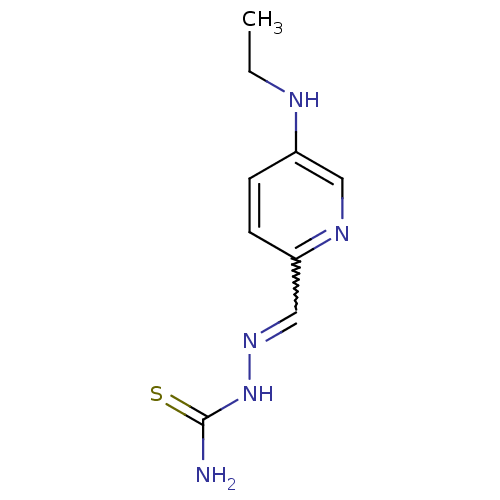
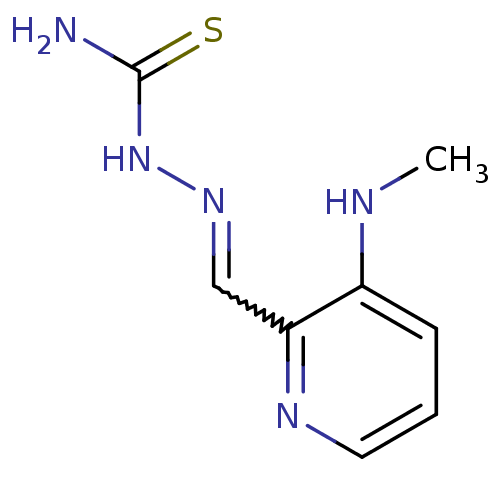

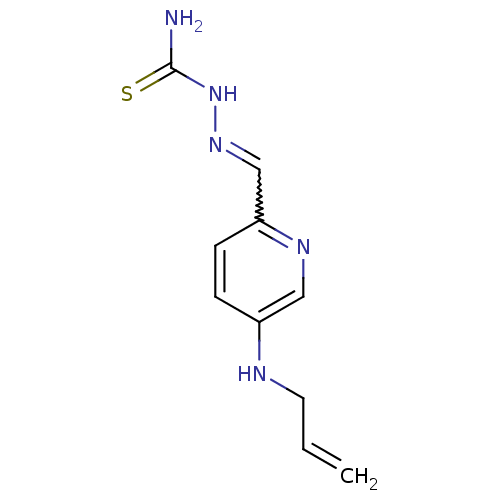
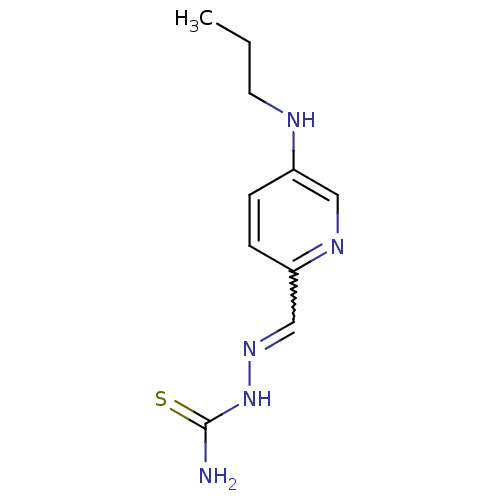
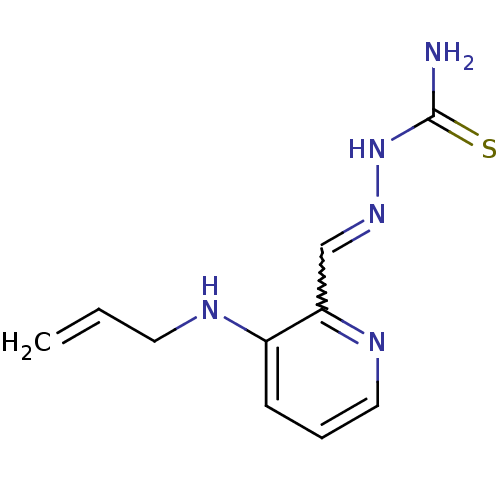
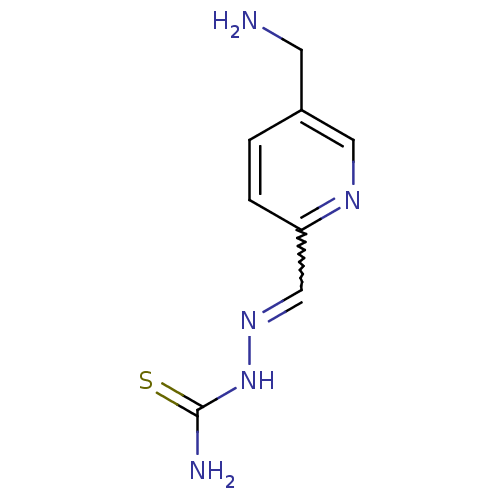
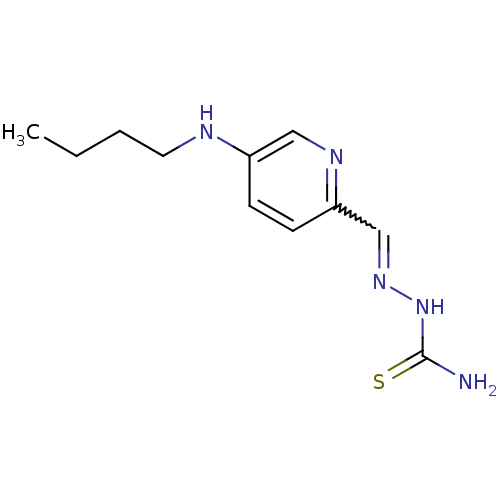
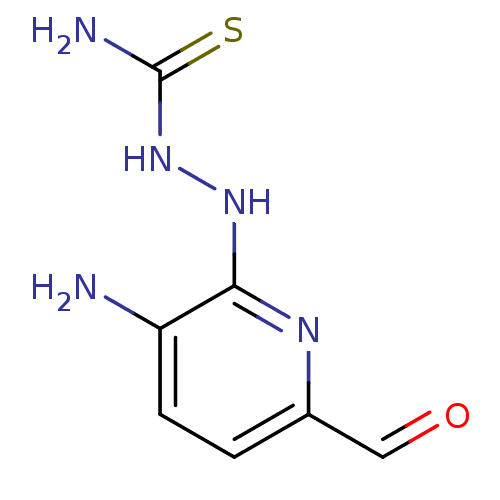

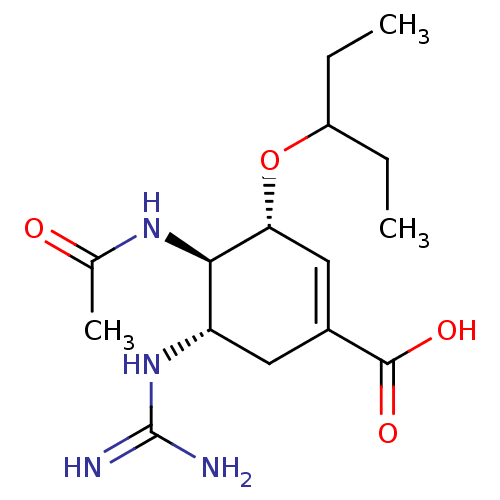
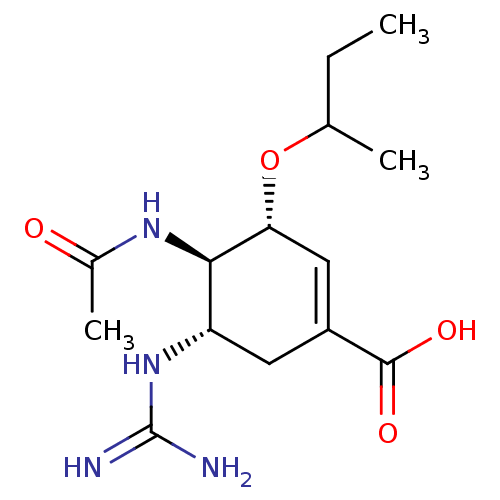

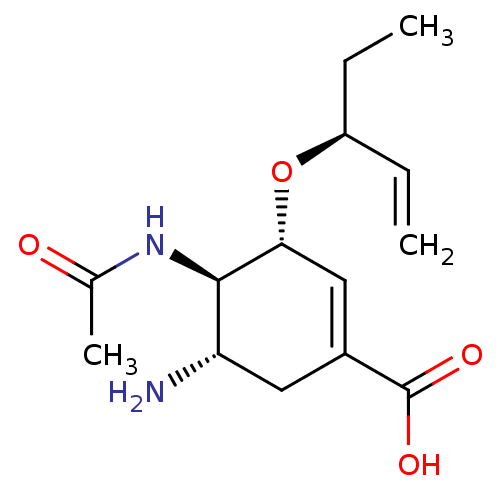
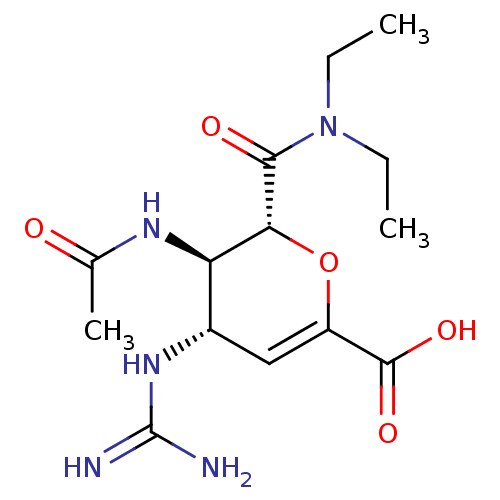

 3D Structure (crystal)
3D Structure (crystal)