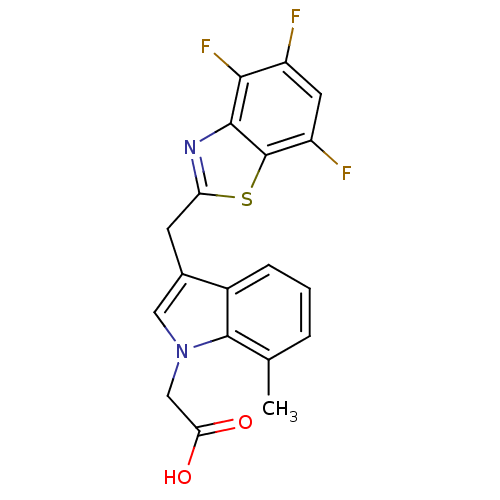
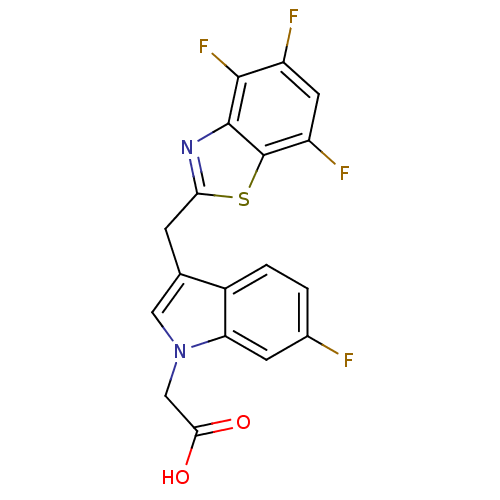
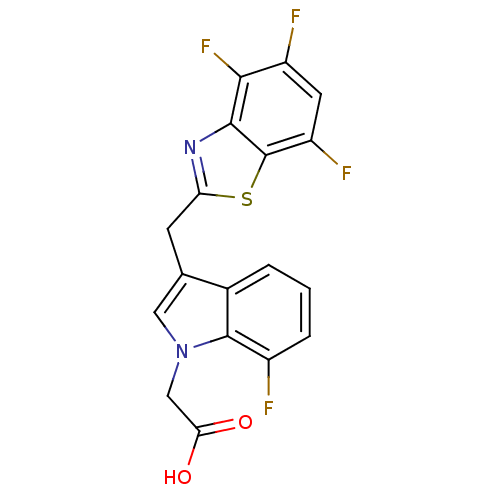
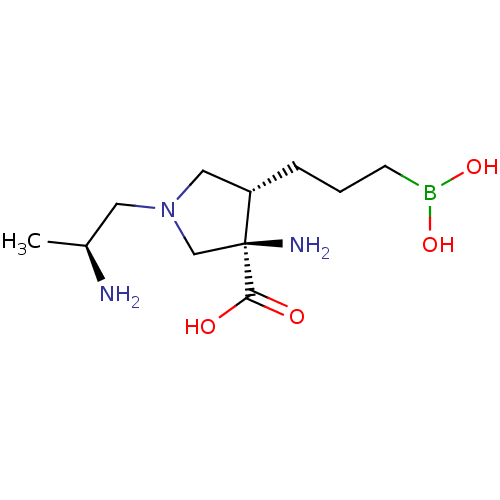
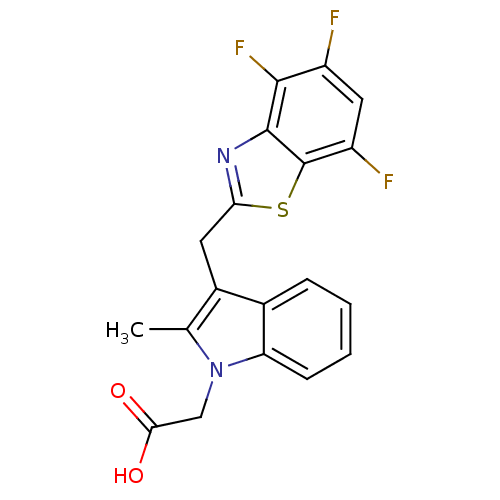
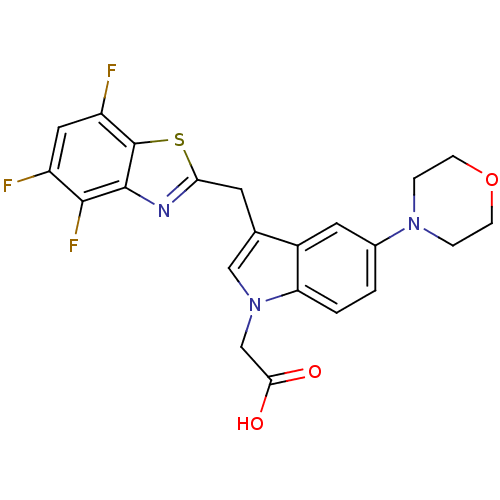
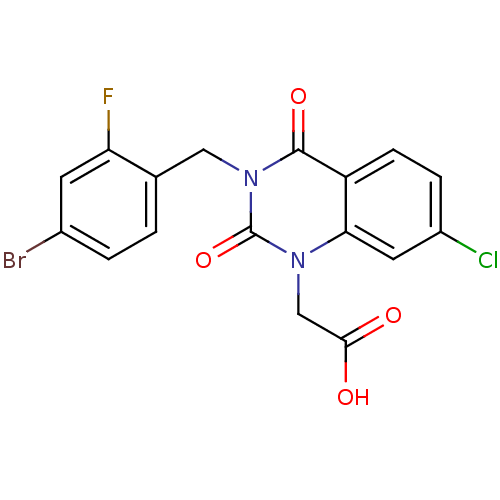
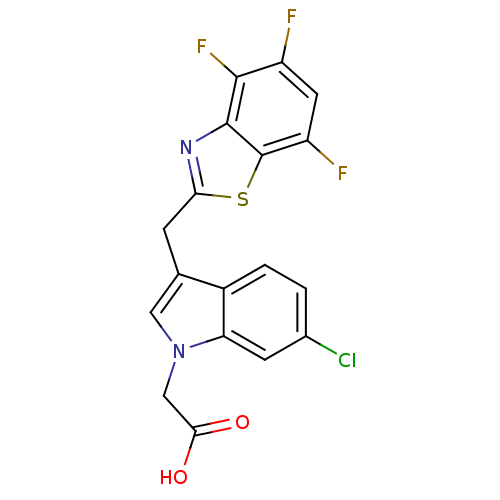
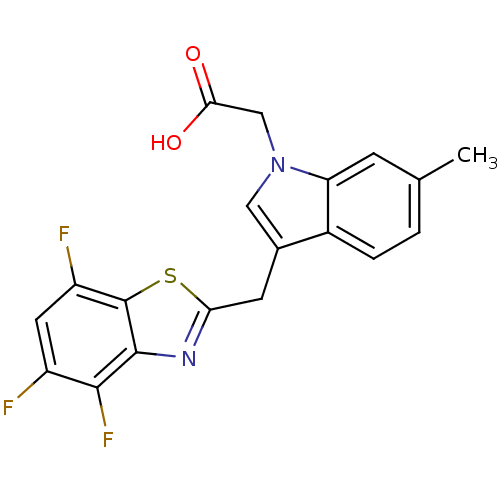
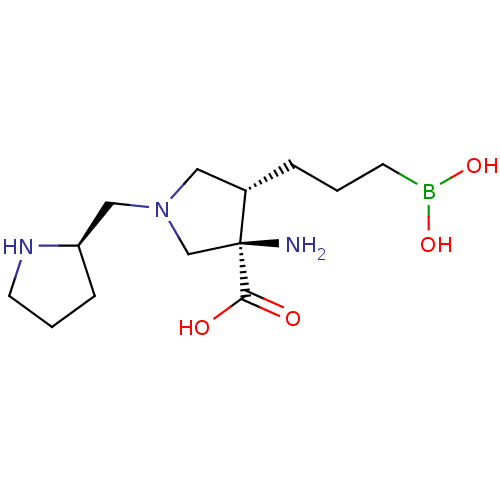
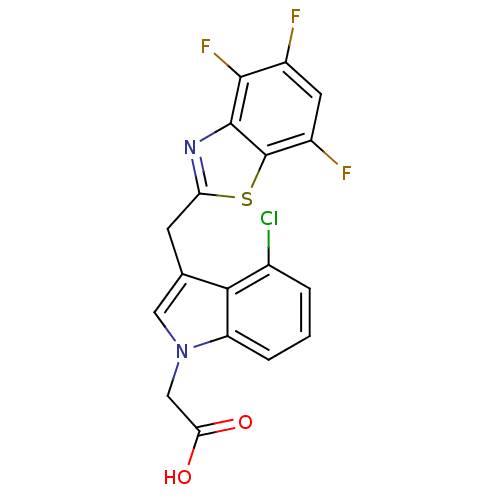
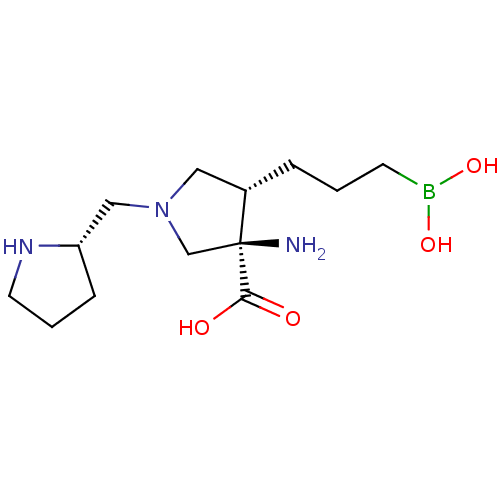
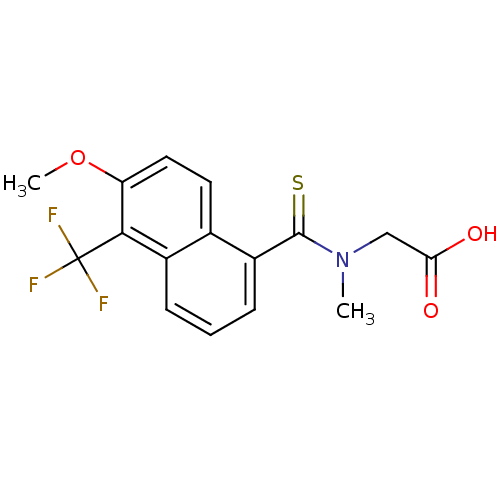
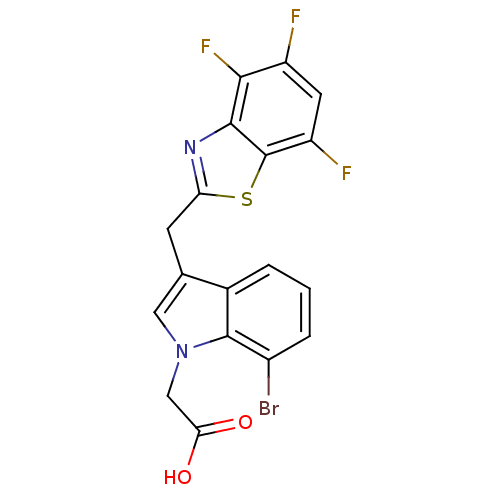
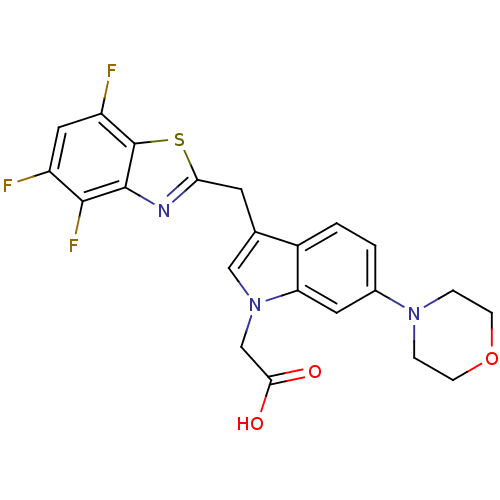
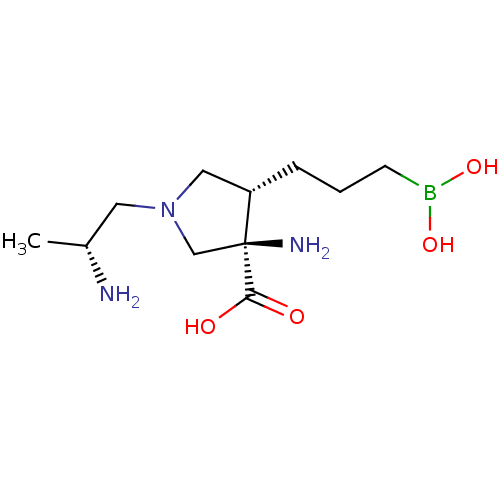
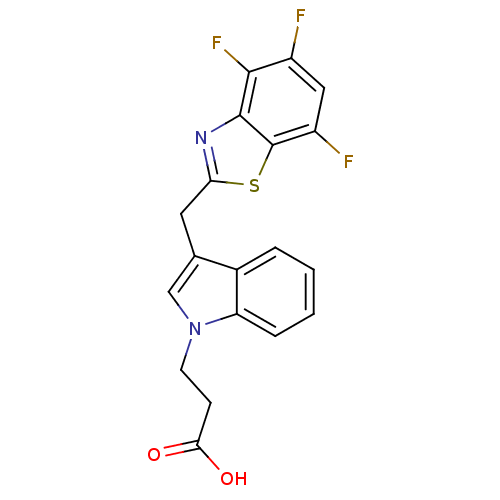
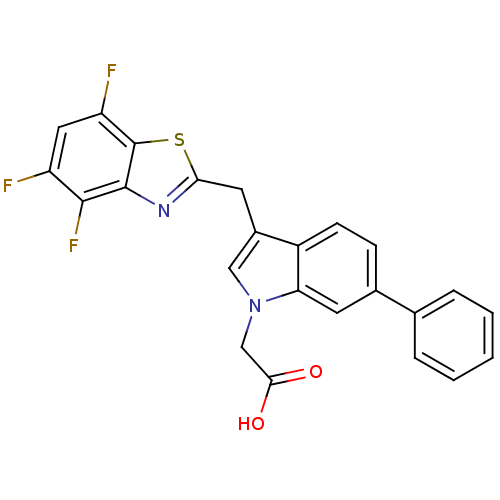
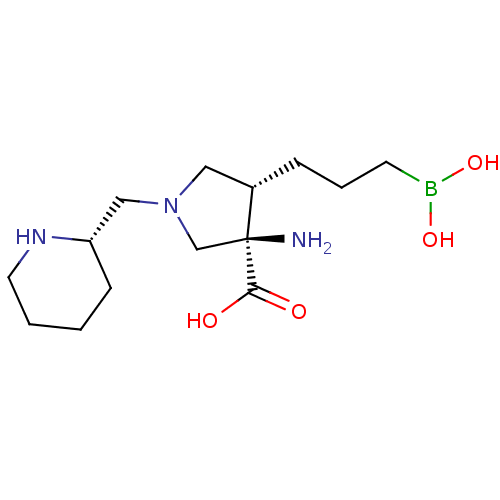
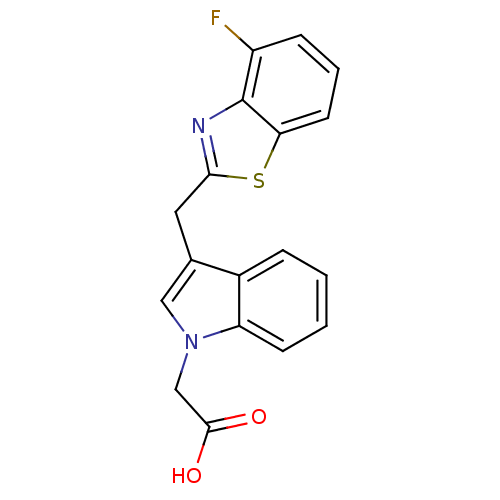
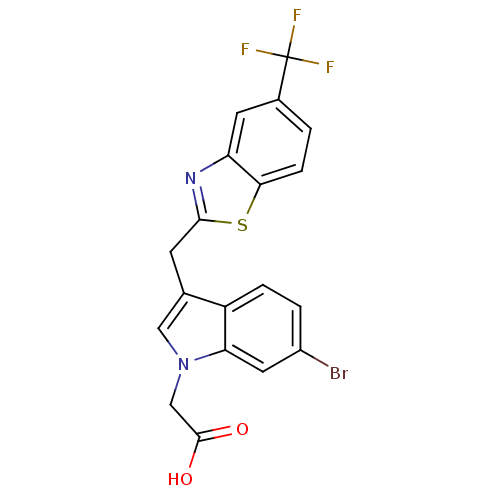
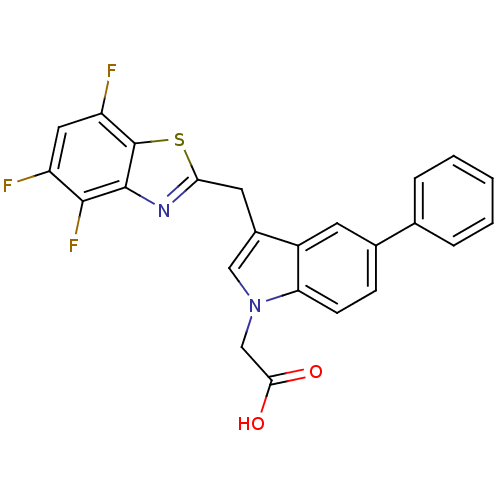
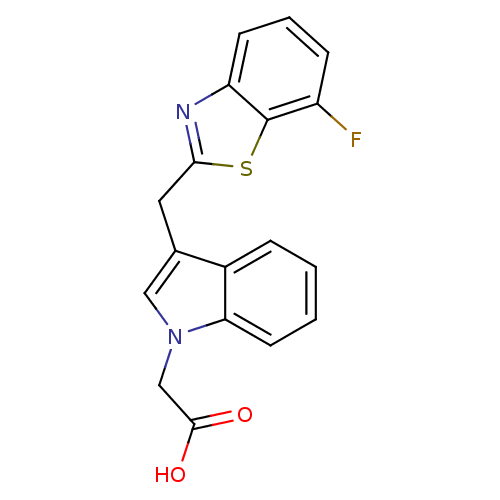
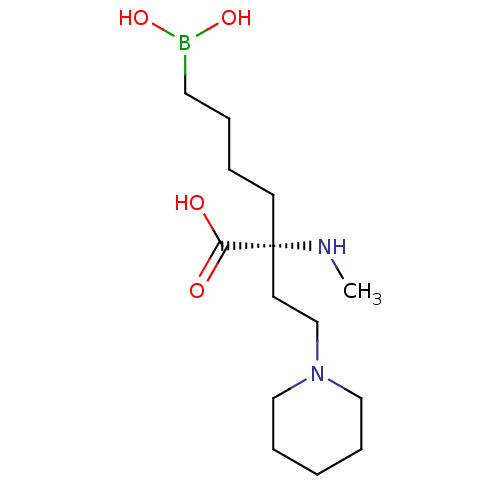
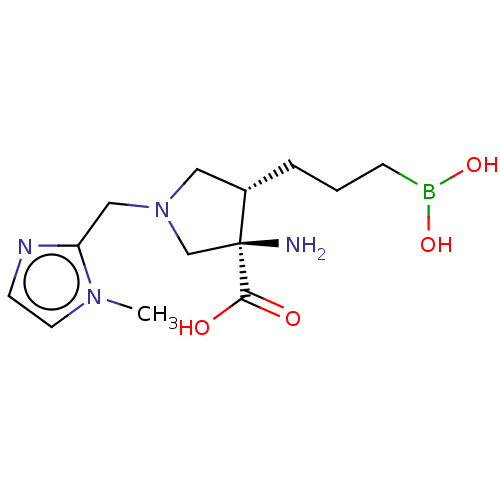
Affinity DataKi: 1.80E+3nMpH: 8.5Assay Description:Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMpH: 8.5Assay Description:Briefly, 0.5-50 mM L-arginine (pH 8.5) was added to a solution of 50 mM 4-(2-hydroxyethyl)piperazine-1-propanesulfonic acid (EPPS) (pH 8.5) and 100 &...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 5nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 5nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 6nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 7nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 7nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 7.70nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 8nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 9nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 9nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 9nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 10nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 10nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 11nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 11nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 12nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 13nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 13nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 14nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 15nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 17nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 25nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 30nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 34nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 52nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 53nMAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 55nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ...More data for this Ligand-Target Pair
TargetArginase-2, mitochondrial(Homo sapiens (Human))
Institutes for Pharmaceutical Discovery , LLC
Curated by ChEMBL
Institutes for Pharmaceutical Discovery , LLC
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 99nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
The Institute for Diabetes Discovery
The Institute for Diabetes Discovery
Affinity DataIC50: 100nMpH: 6.6 T: 2°CAssay Description:The activity of the test enzyme was determined spectrophotometrically by monitoring the change in absorbance at 340 nm, which is due to the disappear...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of human recombinant arginase 1 expressed in Escherichia coli BL21 (DE3) assessed as reduction in urea production using L-arginine as subs...More data for this Ligand-Target Pair

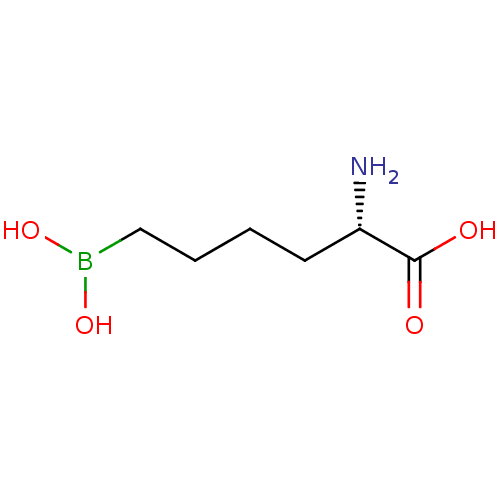
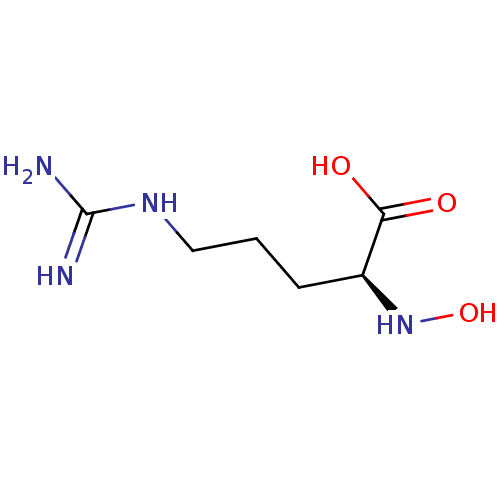

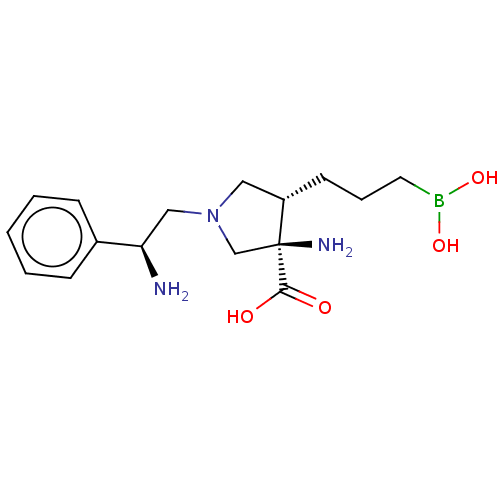


 3D Structure (crystal)
3D Structure (crystal)