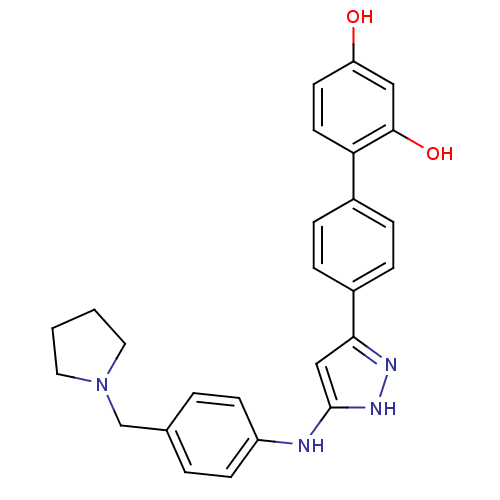
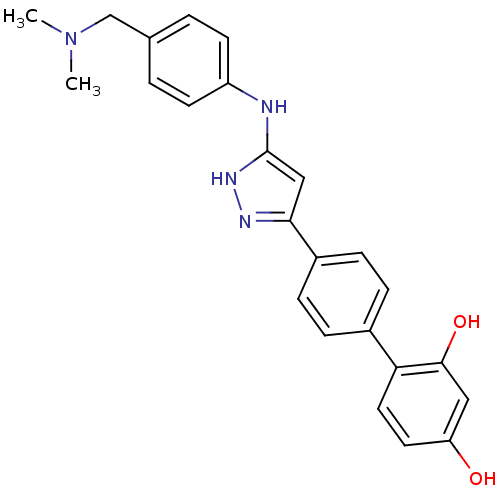
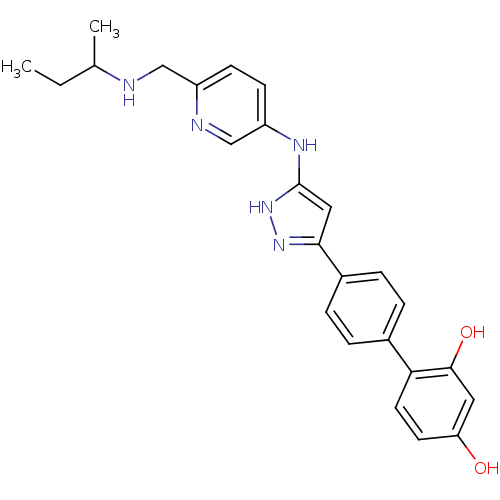
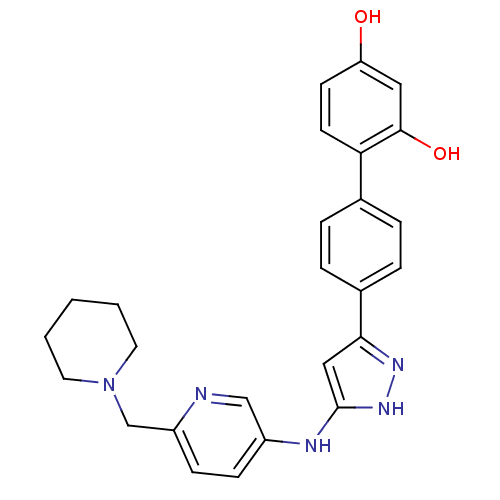
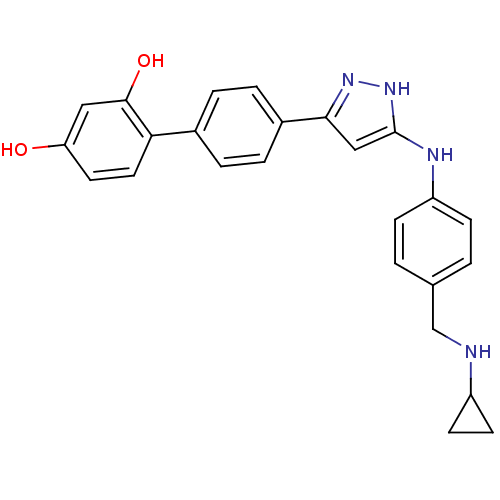
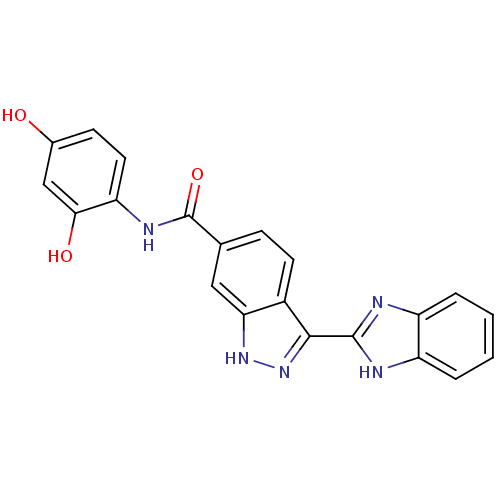
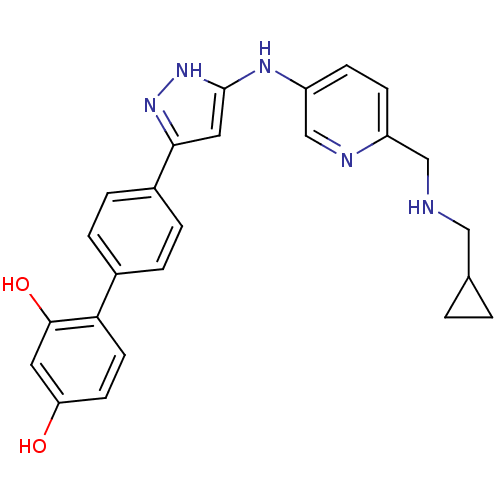
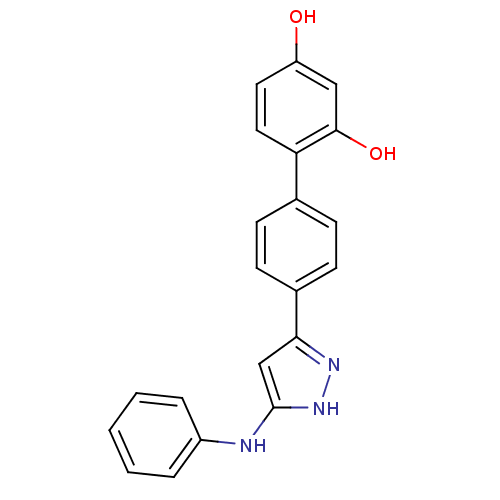
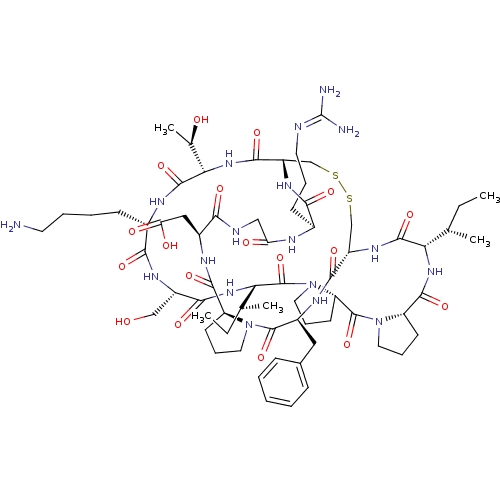
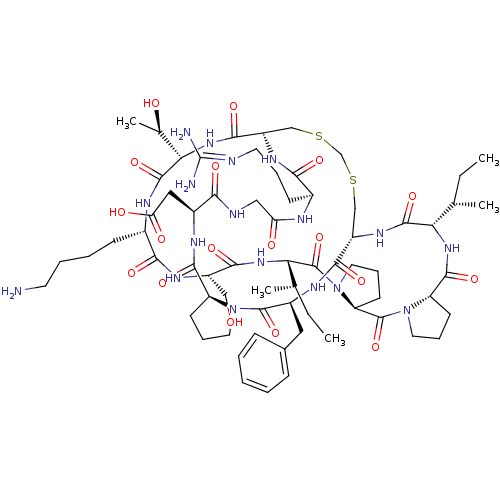
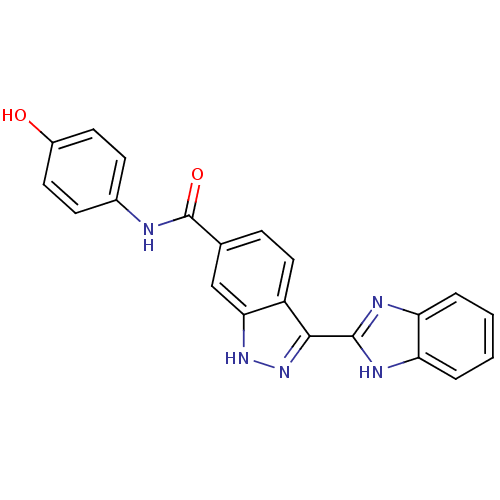
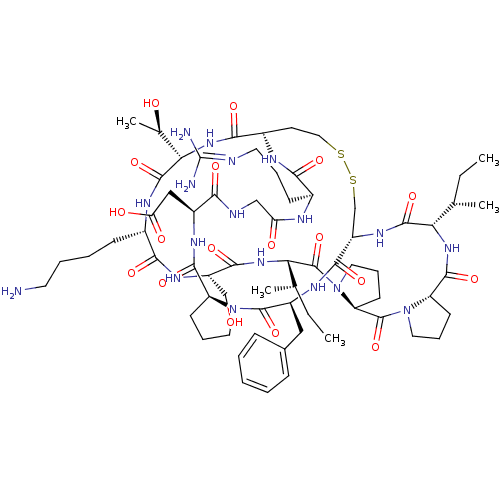
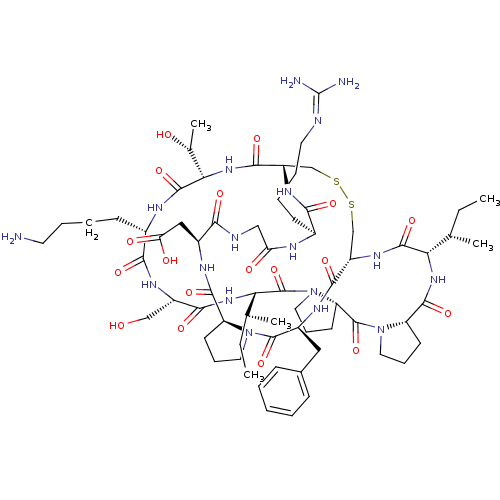
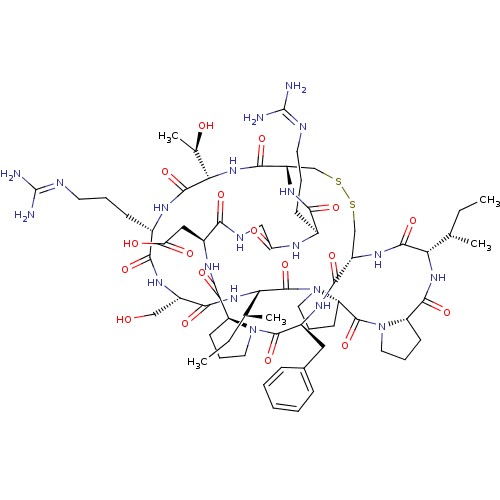
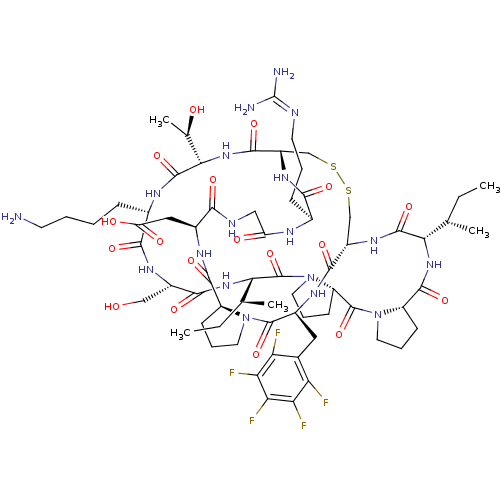
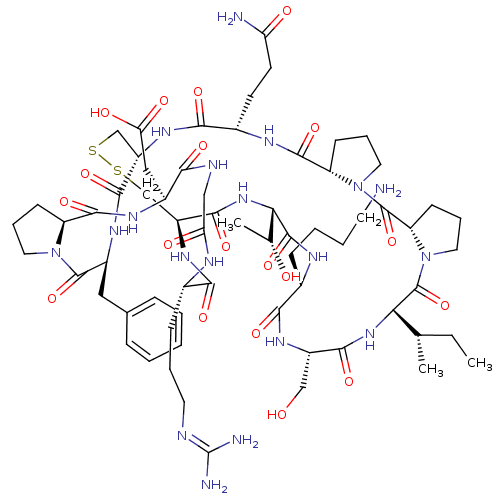
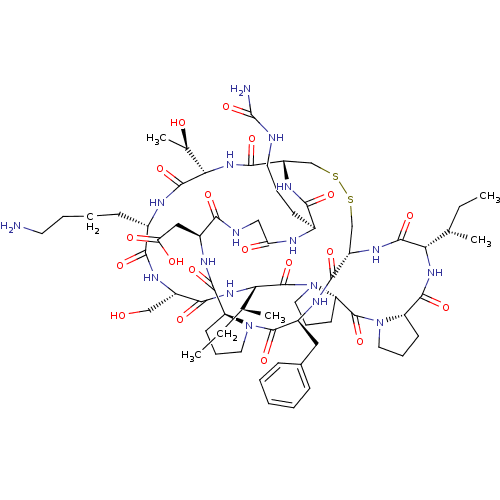
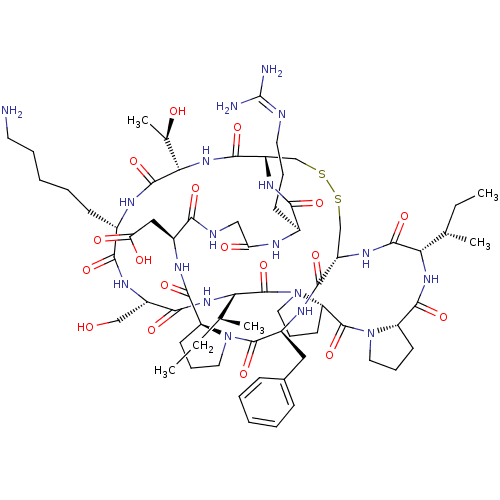
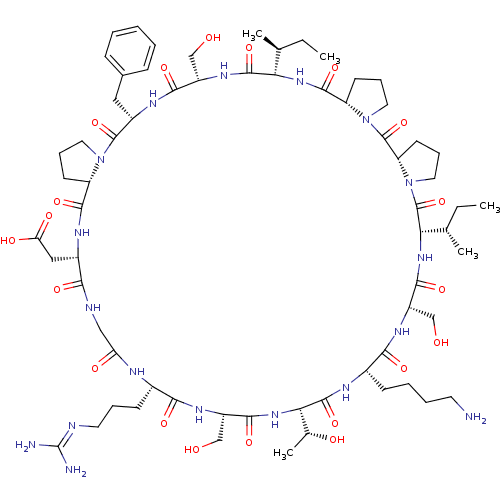
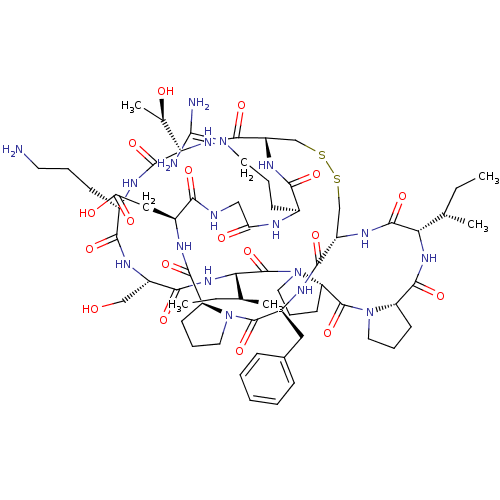
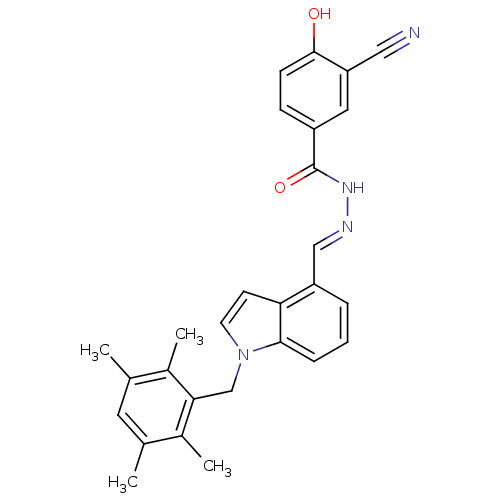
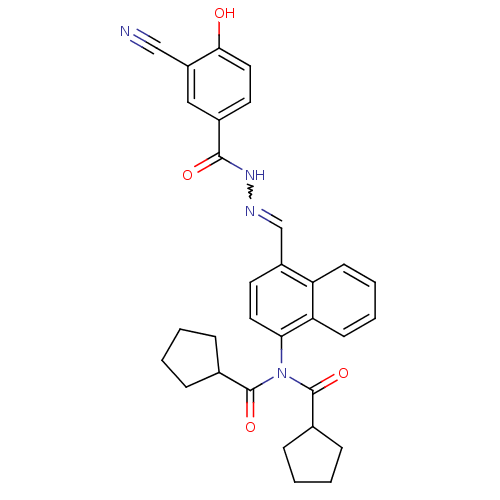
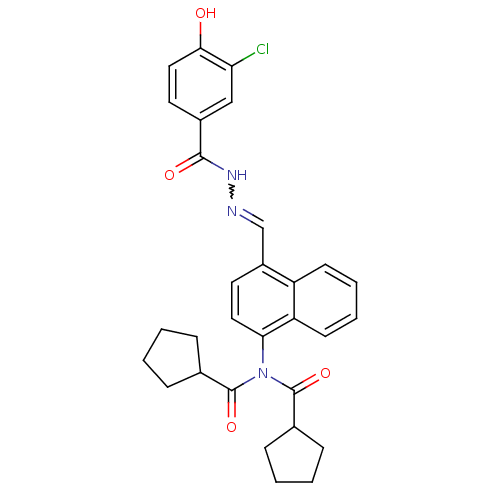
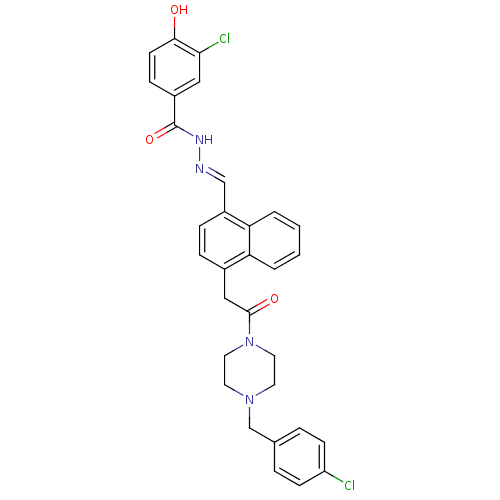
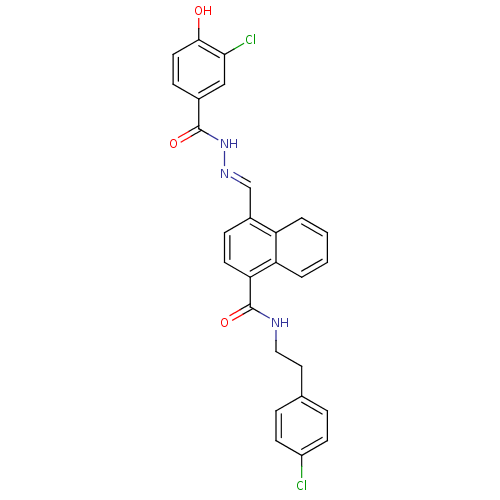
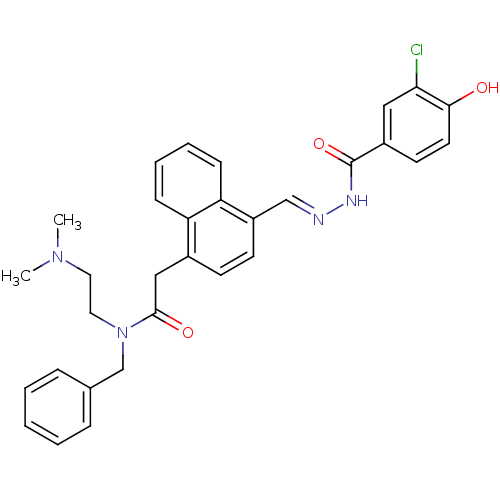
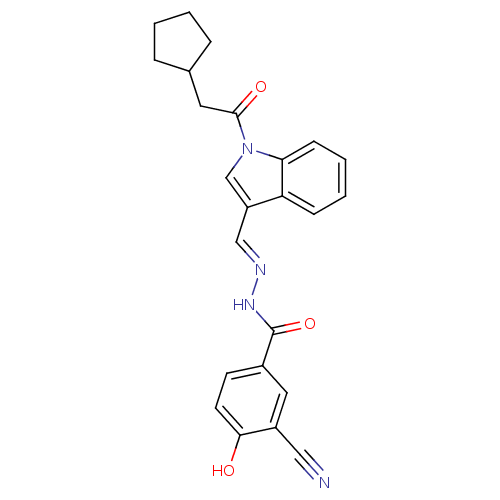
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.180nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.190nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.200nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.360nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.5nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 100nM ΔG°: -39.6kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 160nM ΔG°: -38.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Homo sapiens (Human))
Pfizer Global Research and Development
Curated by ChEMBL
Pfizer Global Research and Development
Curated by ChEMBL
Affinity DataKi: 301nMAssay Description:Inhibition of human CHK1 expressed in baculovirus/insect cell systemMore data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 380nM ΔG°: -36.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 450nM ΔG°: -35.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 857nM ΔG°: -34.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 860nM ΔG°: -34.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 890nM ΔG°: -34.2kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 1.00E+3nM ΔG°: -33.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 2.33E+3nM ΔG°: -31.8kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 4.75E+3nM ΔG°: -30.1kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nM ΔG°: -30.0kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 7.63E+3nM ΔG°: -28.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nM ΔG°: -28.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.63E+4nM ΔG°: -27.1kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 2.25E+4nM ΔG°: -26.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 2.56E+4nM ΔG°: -25.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 2.80E+4nM ΔG°: -25.7kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 3.36E+4nM ΔG°: -25.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 4.04E+4nM ΔG°: -24.8kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 4.56E+4nM ΔG°: -24.5kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 9.00E+4nM ΔG°: -22.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: 1.07E+5nM ΔG°: -22.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.64E+5nM ΔG°: -21.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
TargetSuppressor of tumorigenicity 14 protein(Homo sapiens (Human))
Georgetown University Medical Center
Georgetown University Medical Center
Affinity DataKi: >2.50E+5nM ΔG°: >-20.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 8.27E+5nM ΔG°: -17.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: >2.50E+6nM ΔG°: >-14.7kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:In vitro binding affinity of the compound towards rat glucagon receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.10nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60nMAssay Description:In vitro binding affinity for recombinant human glucagon receptor (hGGR) in BHK cellsMore data for this Ligand-Target Pair