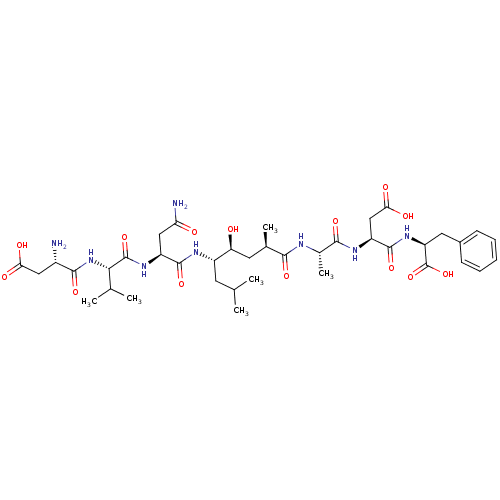
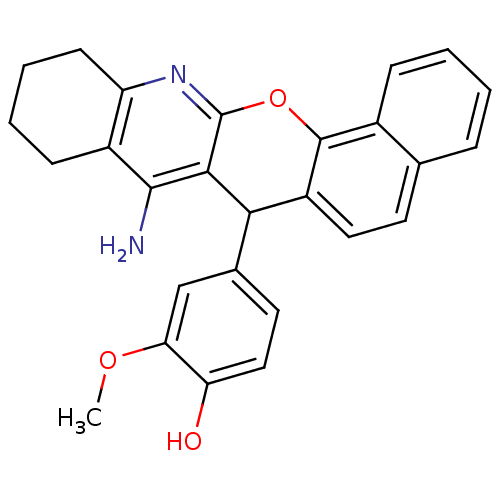
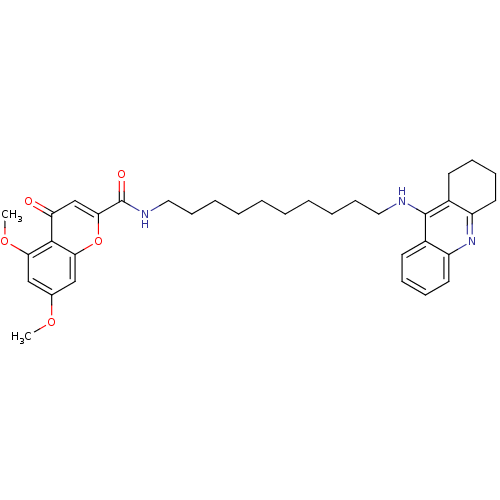
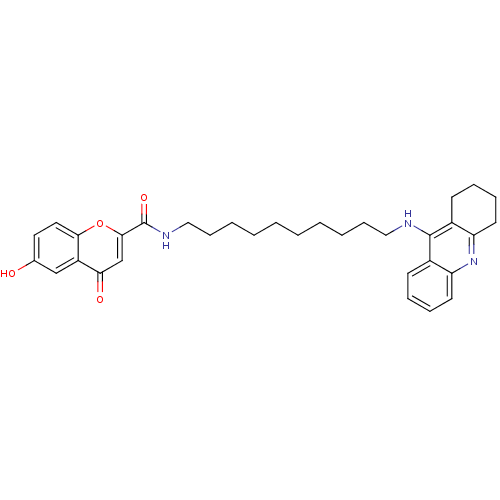
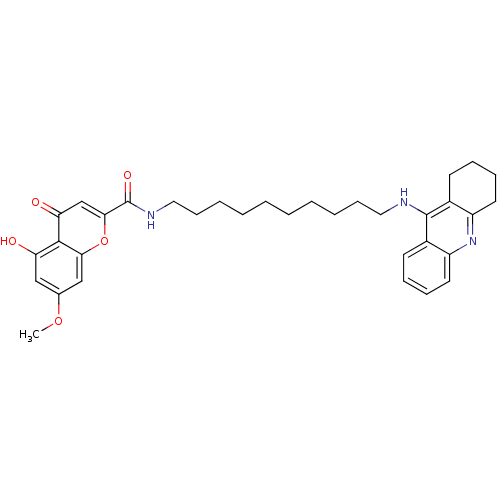
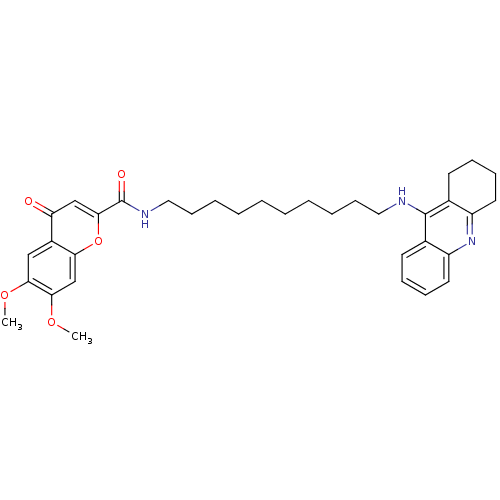
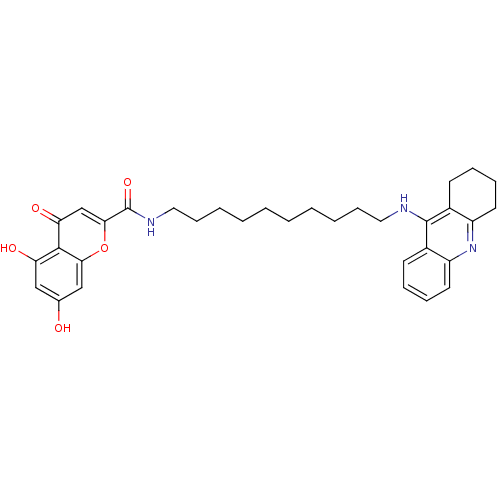
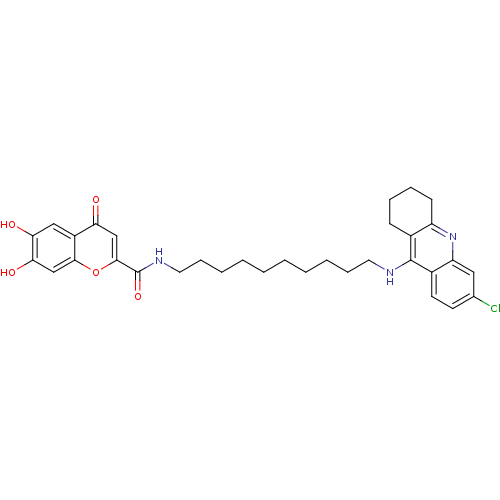
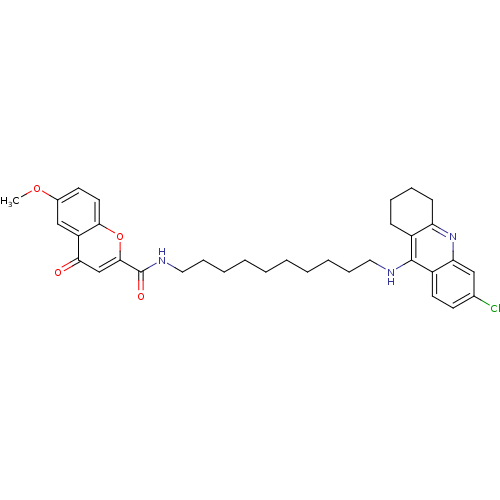
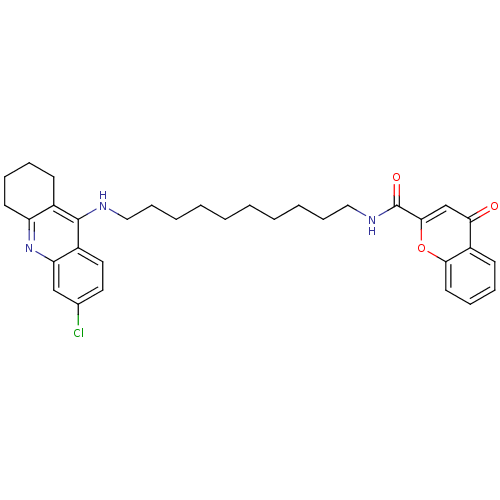
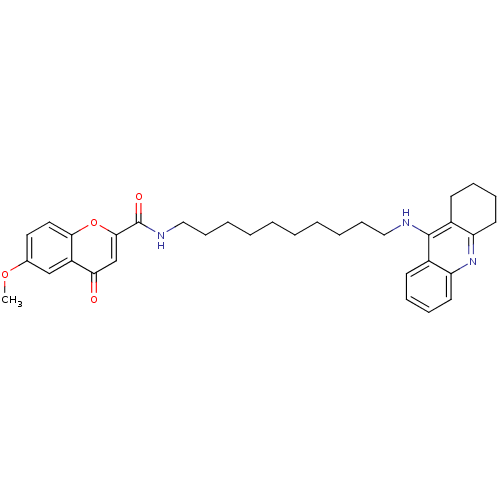
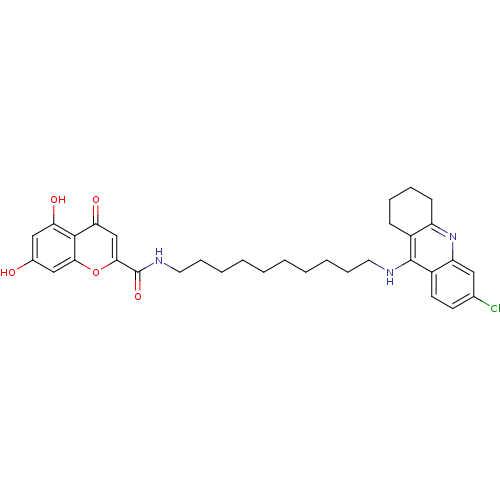
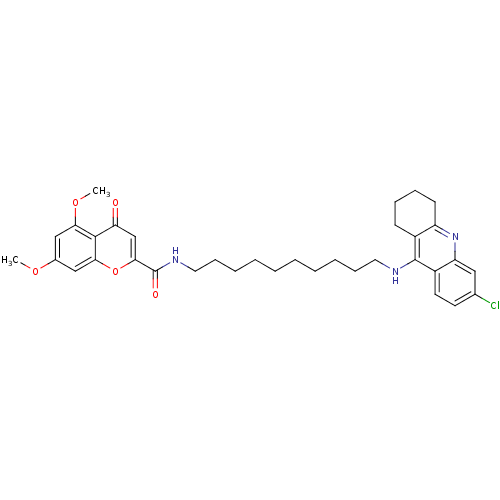
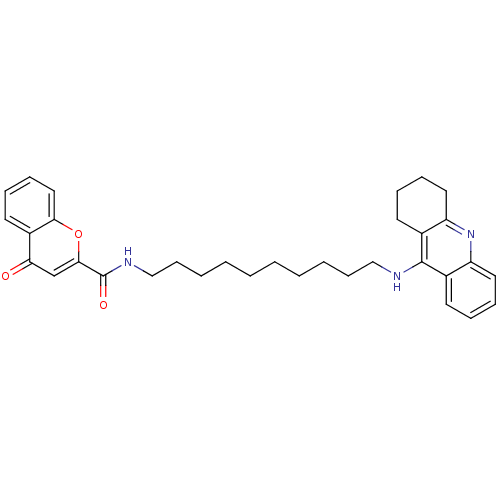
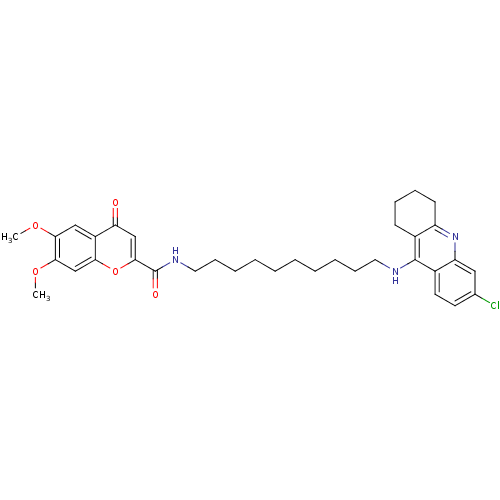
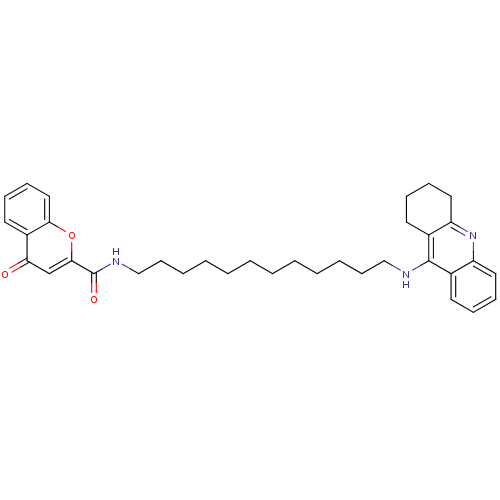
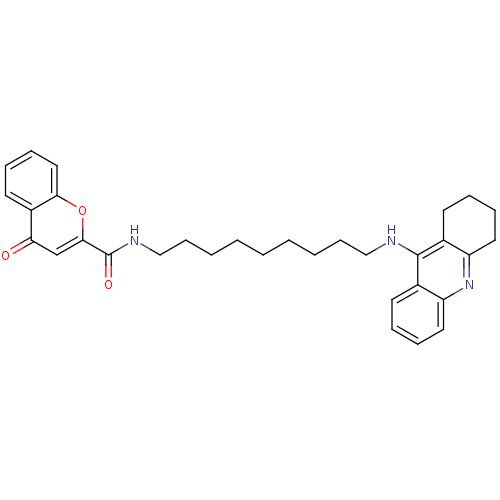
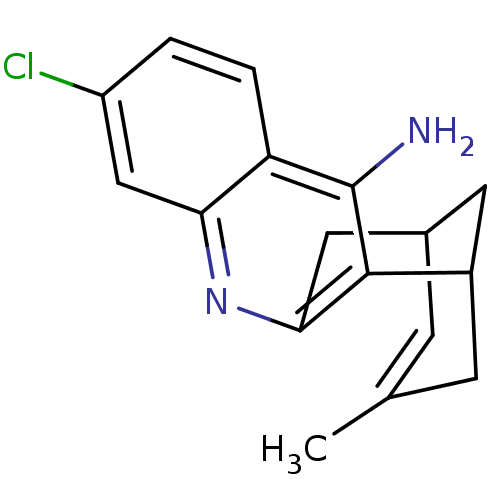
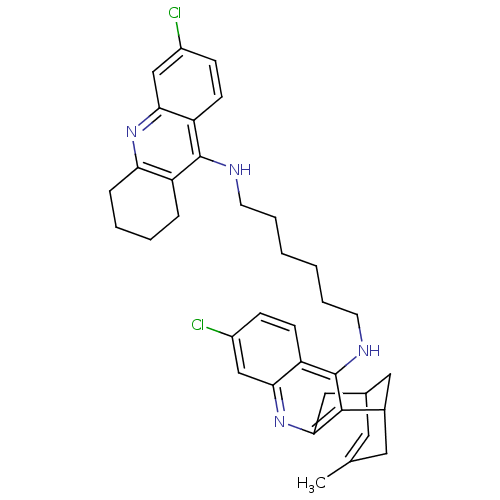
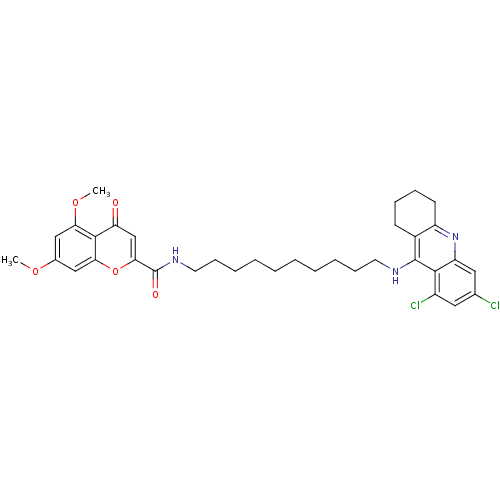
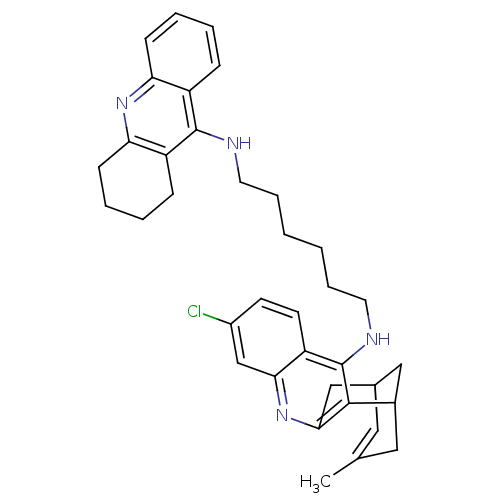
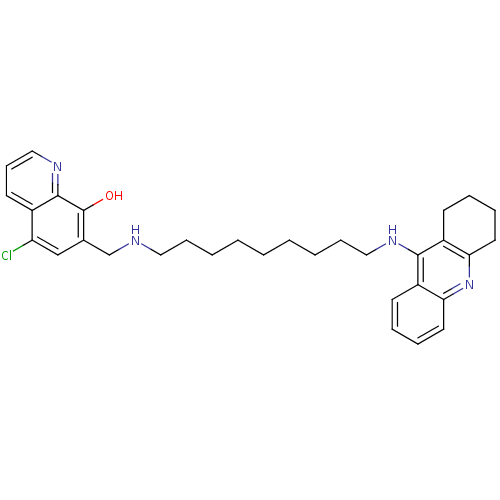
Affinity DataKi: 1.5nMAssay Description:Competitive inhibition of human recombinant AChE by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 9.70nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Universit£ de Sfax
Curated by ChEMBL
Universit£ de Sfax
Curated by ChEMBL
Affinity DataKi: 81nMAssay Description:Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataKi: 620nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 820nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 850nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 880nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.06E+3nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.99E+3nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.23E+3nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.58E+3nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.13E+4nMAssay Description:Competitive inhibition of human recombinant BChE using panvera peptide as substrate preincubated for 60 mins before substrate addition by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0350nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0900nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.350nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.350nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.690nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Inhibition of horse serum BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human erythrocyte AChE by Ellman's reactionMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human erythrocytes AChE by Ellman's methodMore data for this Ligand-Target Pair