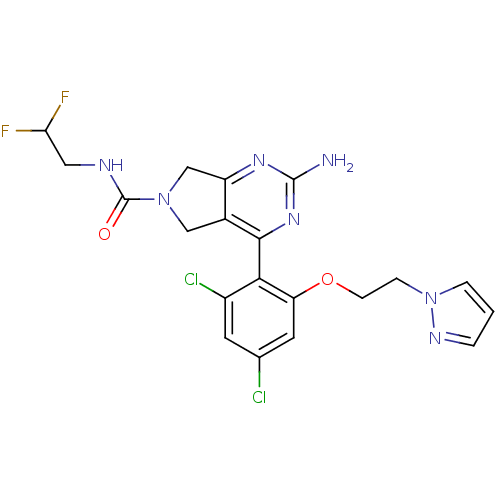
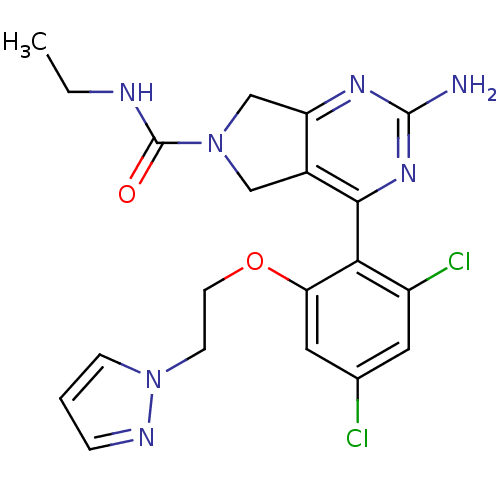
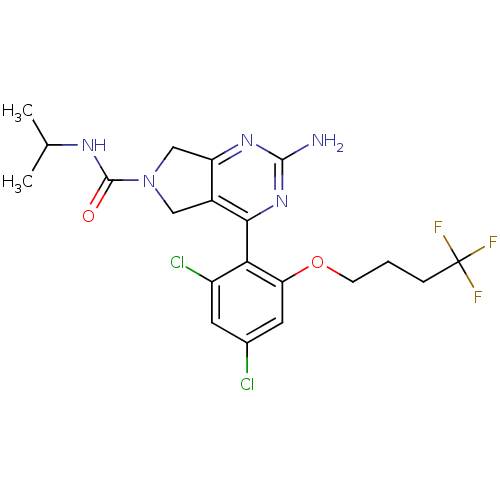
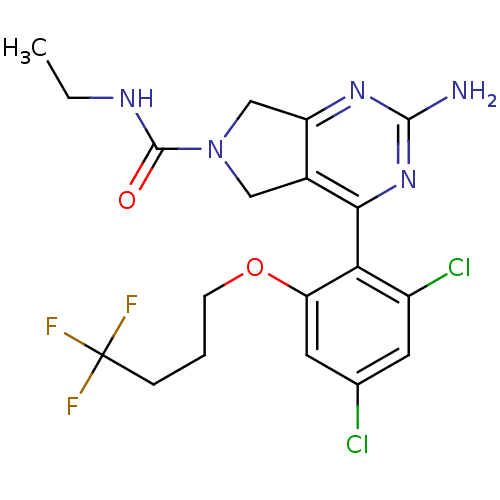
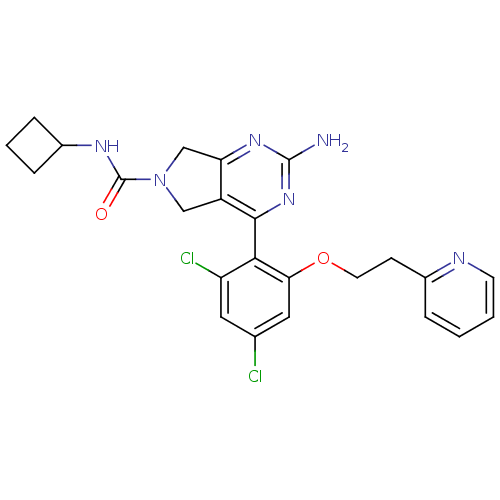
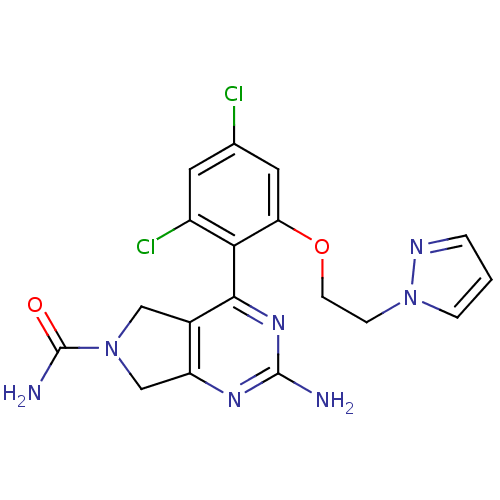
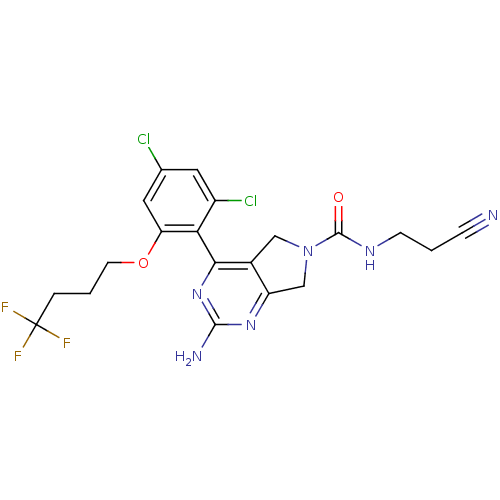
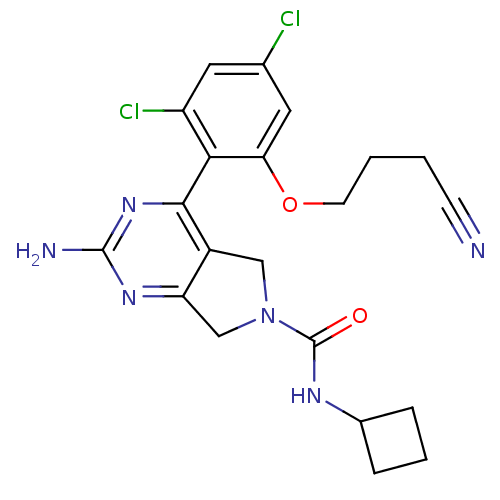
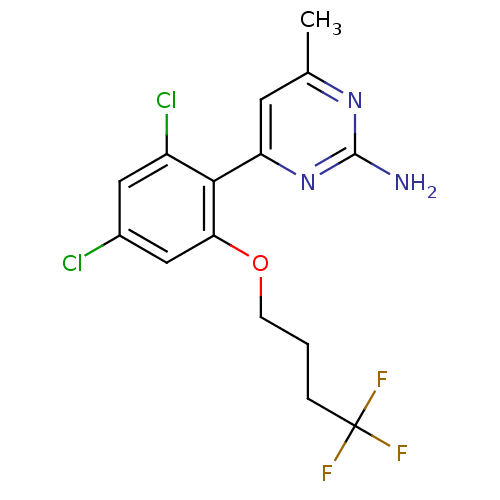
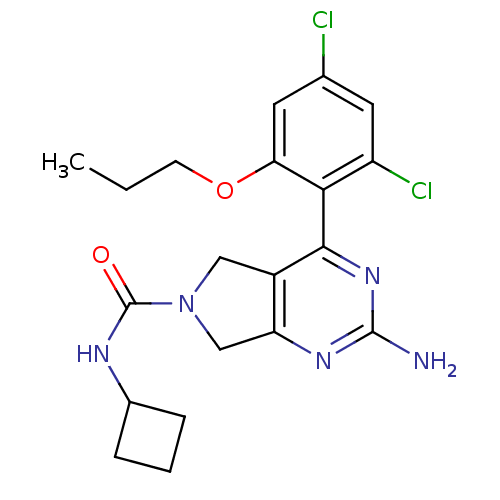
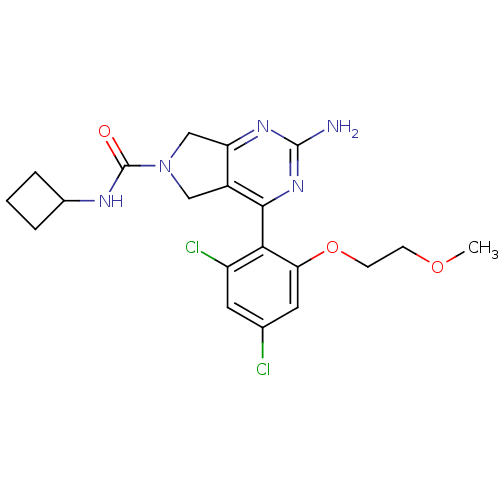
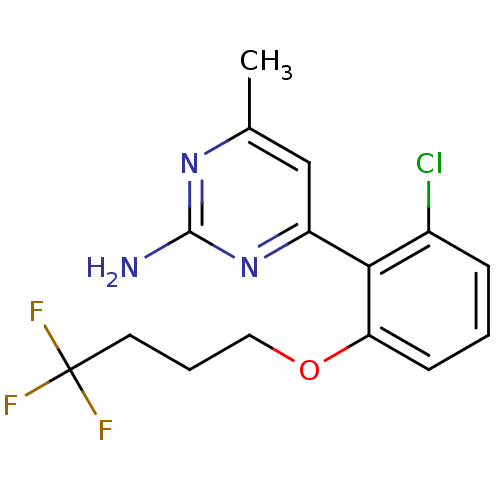
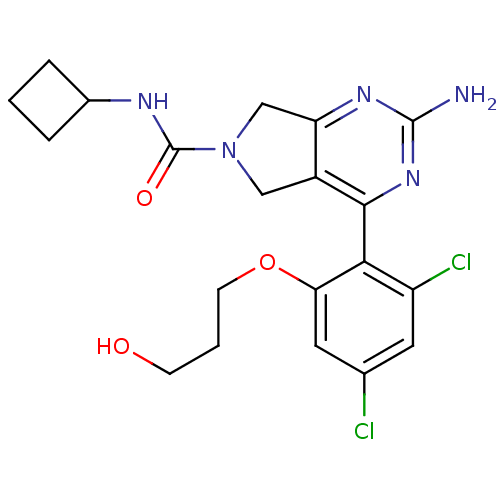
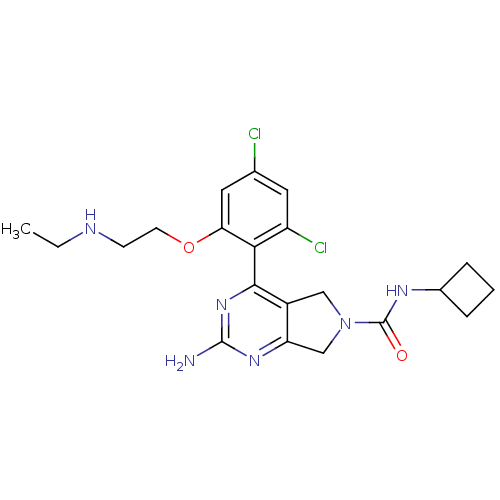
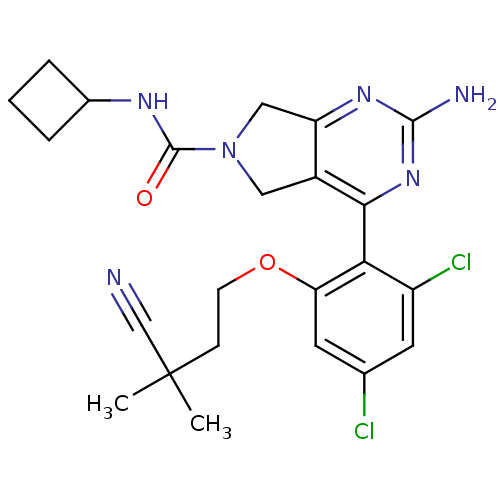
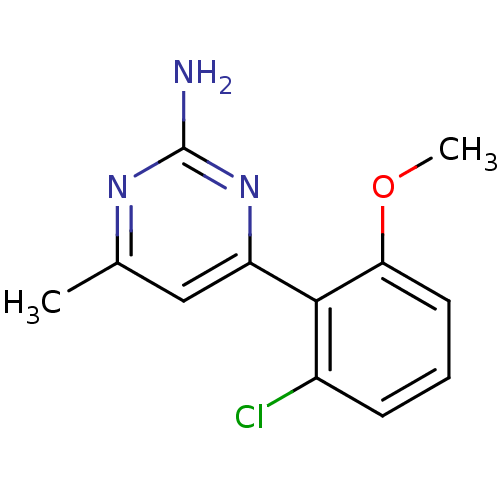
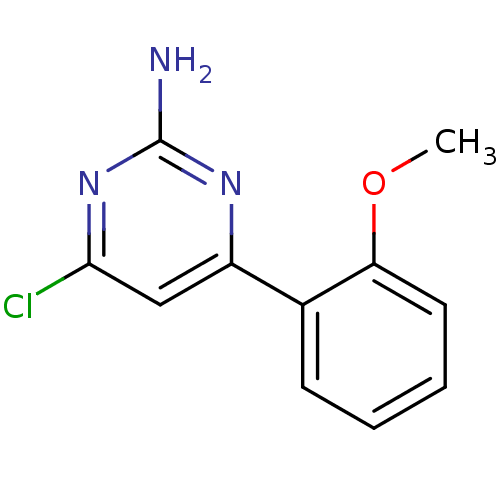
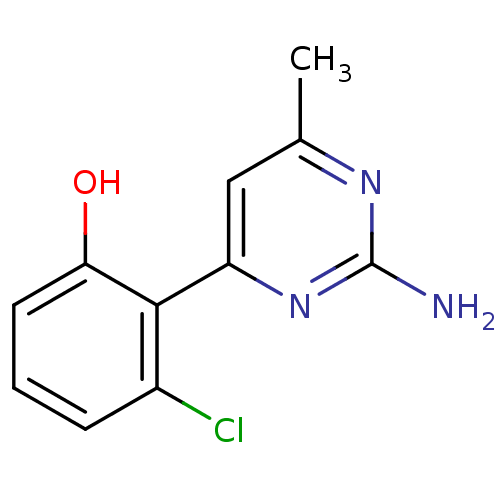
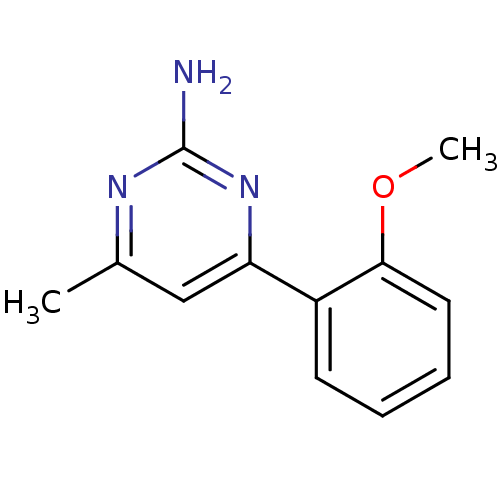
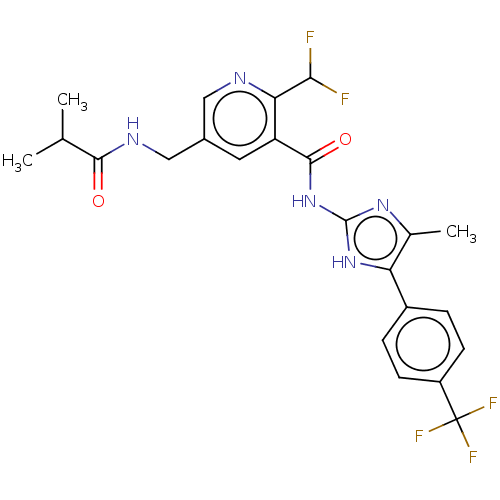
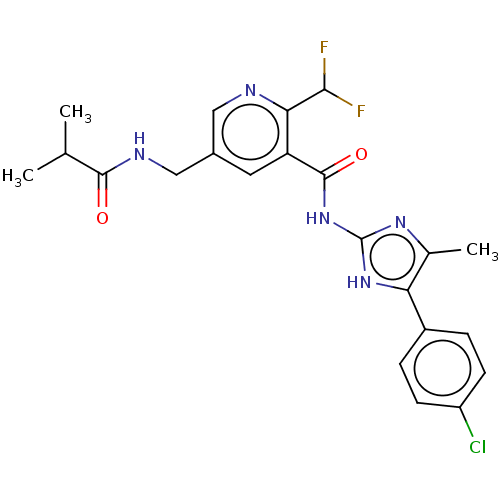
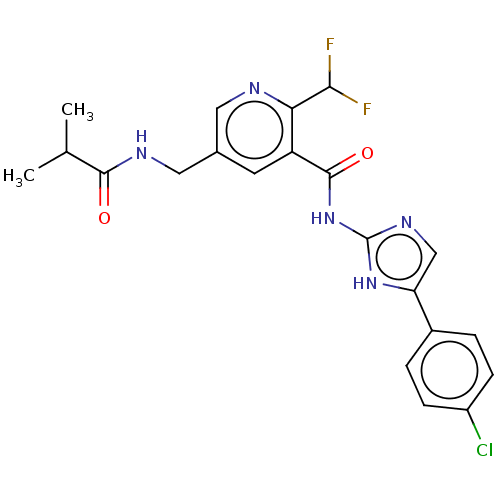
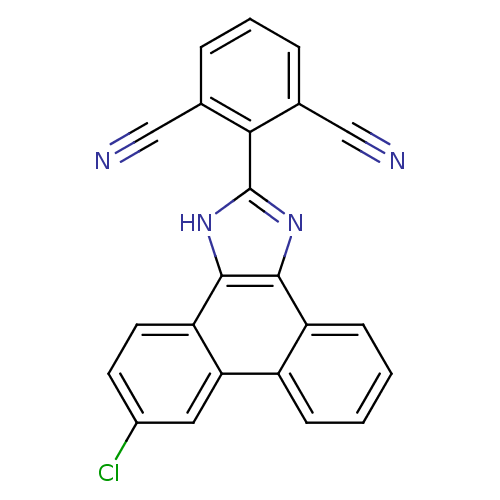
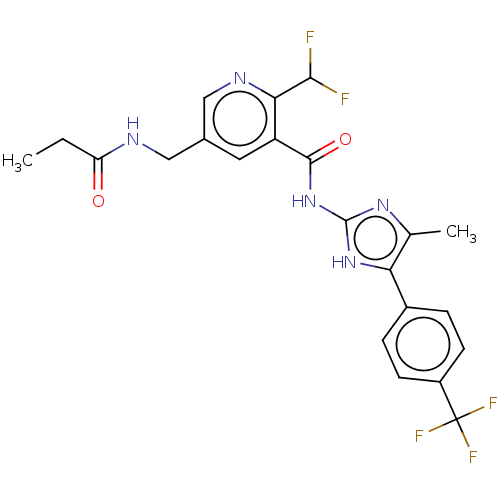
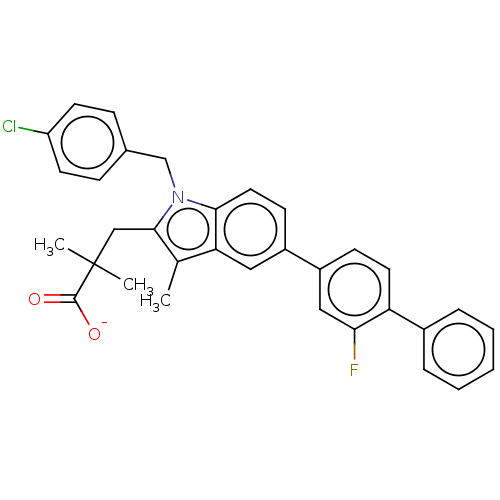
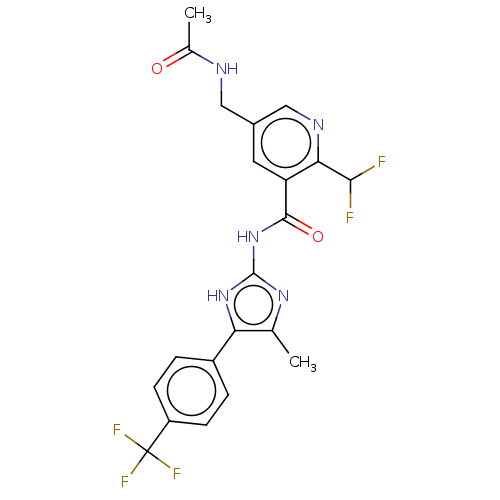
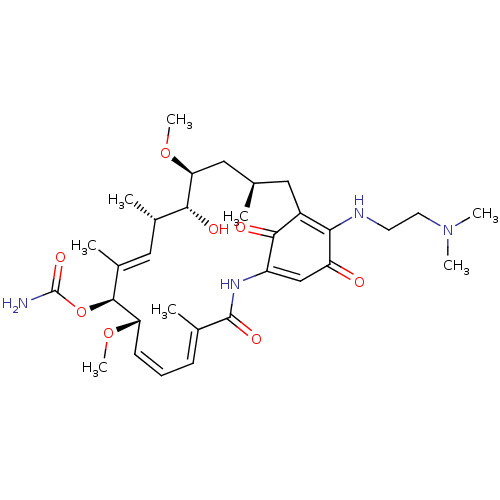
Affinity DataKi: 6nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
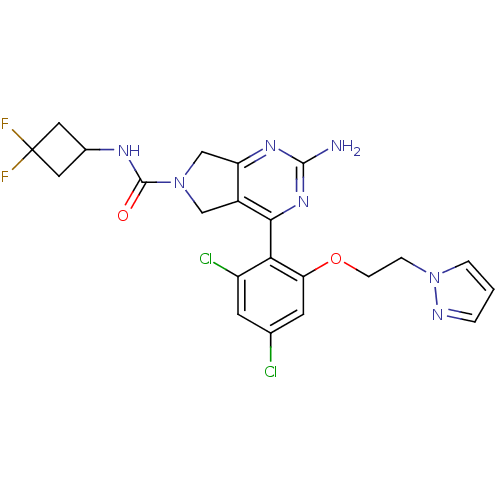
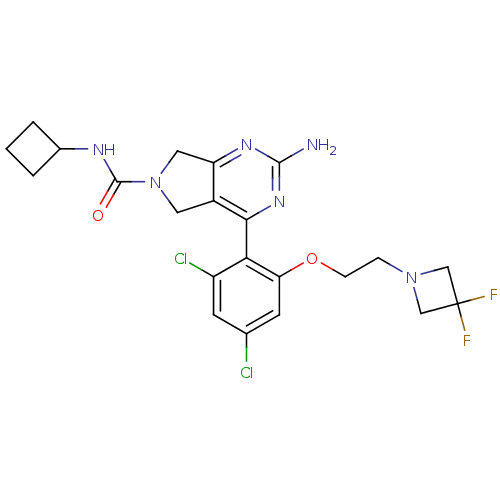
Affinity DataKi: 7nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
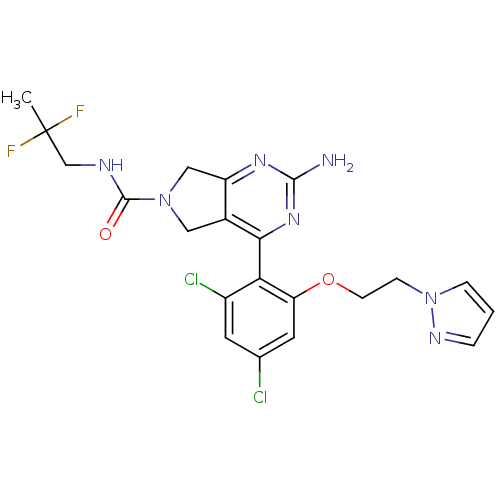
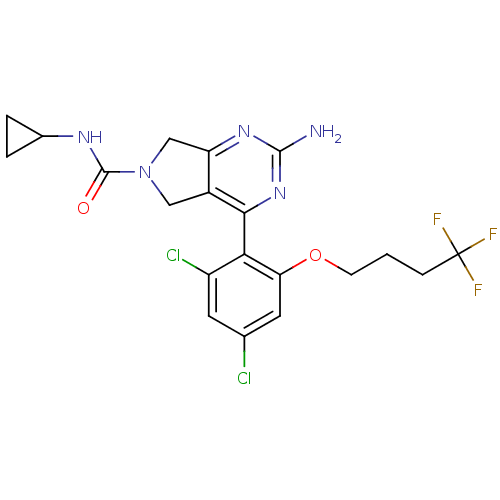
Affinity DataKi: 7nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
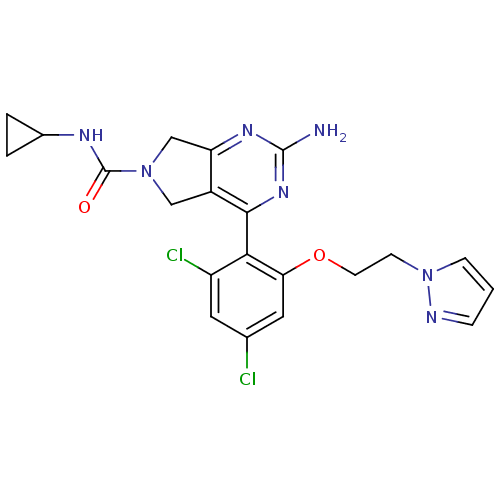
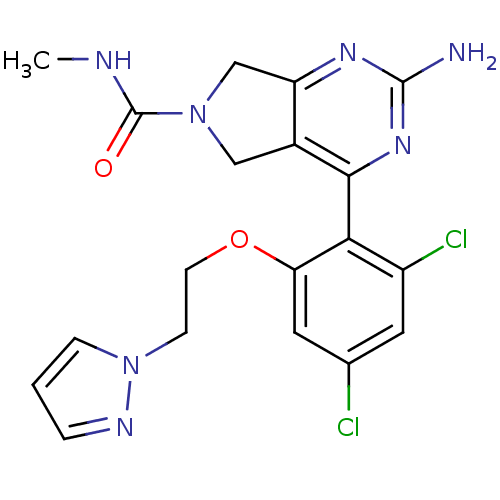
Affinity DataKi: 7nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 50nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 120nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 300nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 700nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
Affinity DataKi: 8.30E+3nMAssay Description:Displacement of [3H]-(R)-2-(5-chloro-2,4-dihydroxybenzoyl)-N-ethylisoindoline-1-carboxamide from human his(6)-tagged HSP90alpha after 30 mins by scin...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: 1.16E+4nMAssay Description:Displacement of [3H]dofetilide from human ERG channel expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Pfizer Inc.
Curated by ChEMBL
Pfizer Inc.
Curated by ChEMBL
Affinity DataKi: 4.13E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of mPGES1 in rhIL-1beta-stimulated human A549 cells assessed as PGE2 level treated for 18 hrs after 30 mins pre-incubation with rhIL-1beta...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of mPGES1 in LPS-induced human whole blood assessed as suppression of PGE2 response after 20 to 24 hrs by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of mPGES1 in rhIL-1beta-stimulated human A549 cells assessed as PGE2 level treated for 18 hrs after 30 mins pre-incubation with rhIL-1beta...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of mPGES1 in LPS-induced human whole blood assessed as suppression of PGE2 response after 20 to 24 hrs by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inhibition of mPGES1 in rhIL-1beta-stimulated human A549 cells assessed as PGE2 level treated for 18 hrs after 30 mins pre-incubation with rhIL-1beta...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of mPGES1 in rhIL-1beta-stimulated human A549 cells assessed as PGE2 level treated for 18 hrs after 30 mins pre-incubation with rhIL-1beta...More data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:Inhibition of mPGES1 in rhIL-1beta-stimulated human A549 cells assessed as PGE2 level treated for 18 hrs after 30 mins pre-incubation with rhIL-1beta...More data for this Ligand-Target Pair
Affinity DataIC50: 91nMAssay Description:Inhibition of mPGES1 in LPS-induced human whole blood assessed as suppression of PGE2 response after 20 to 24 hrs by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inhibition of mPGES1 in rhIL-1beta-stimulated human A549 cells assessed as PGE2 level treated for 18 hrs after 30 mins pre-incubation with rhIL-1beta...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of Hsp90 in human H1299 assessed as degradation of Hsp90 client protein AktMore data for this Ligand-Target Pair

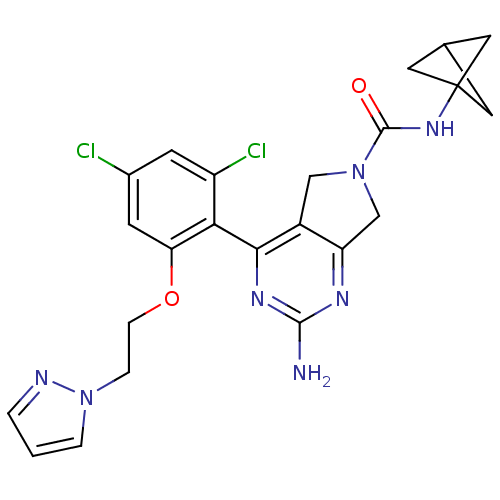
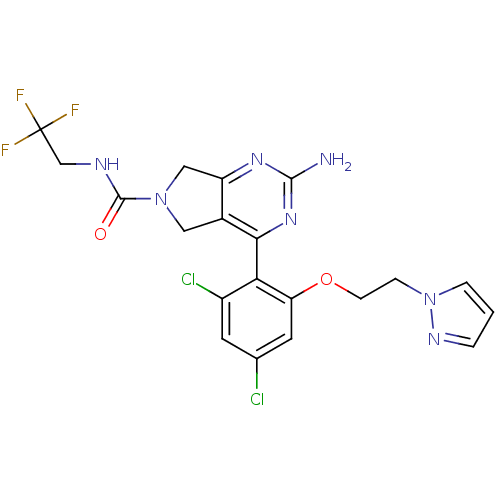
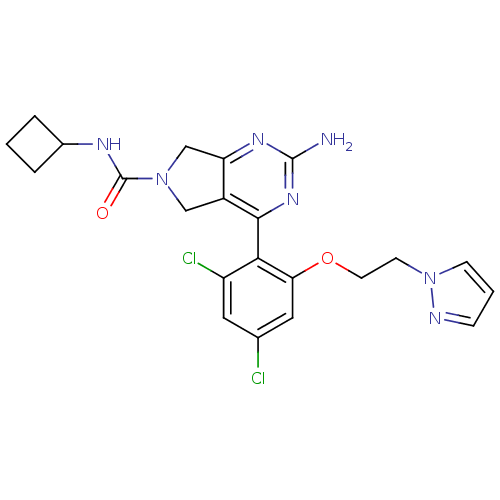
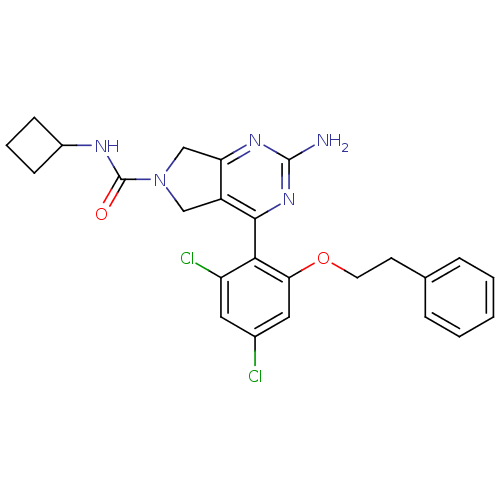
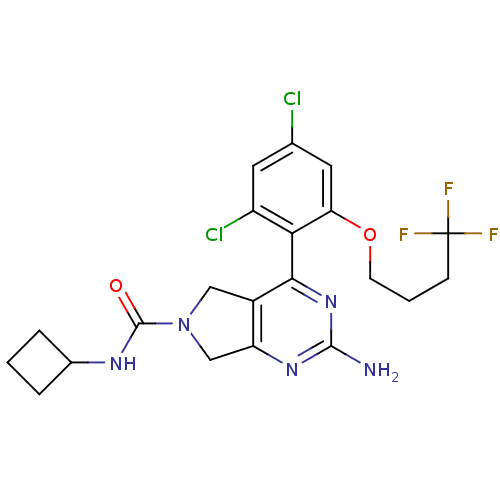
 3D Structure (crystal)
3D Structure (crystal)