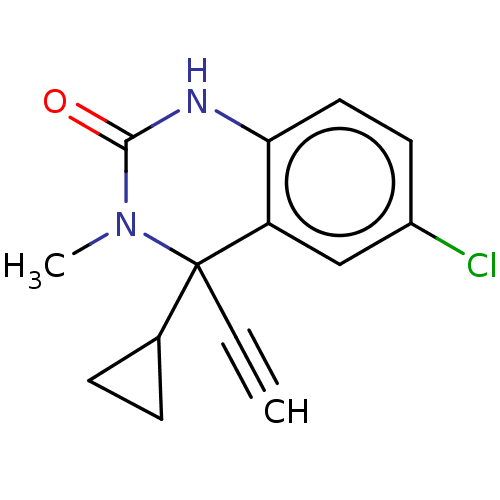
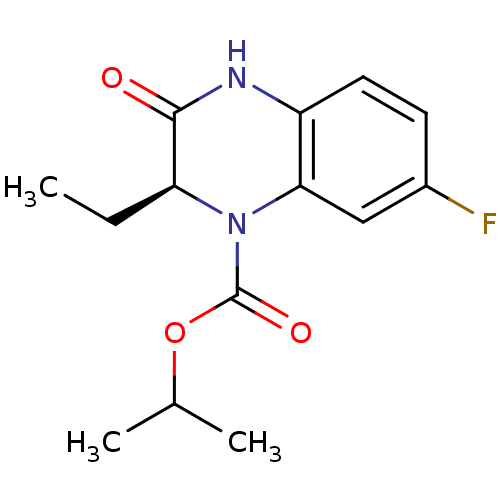
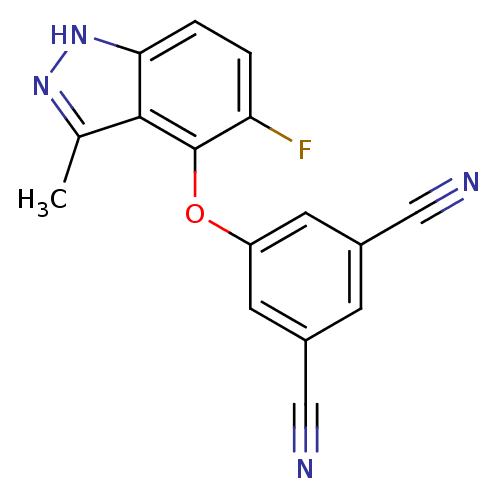
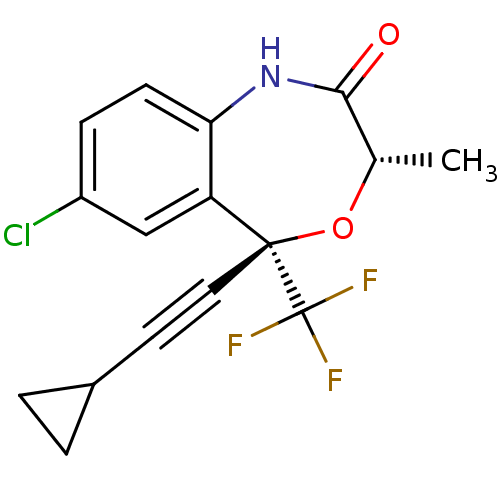
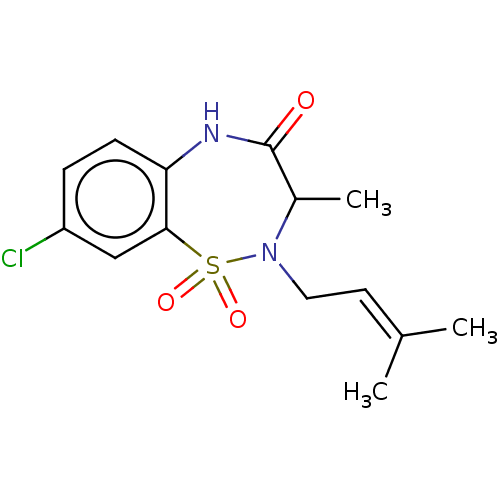
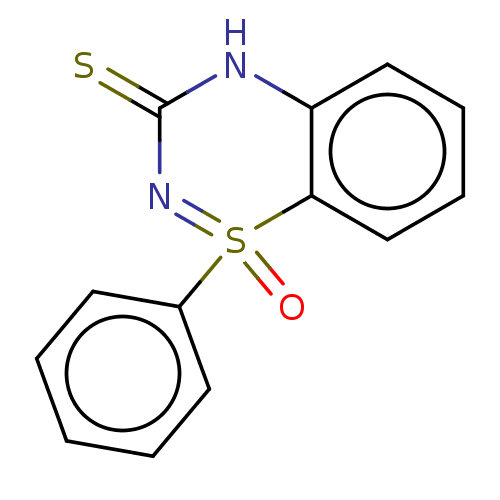
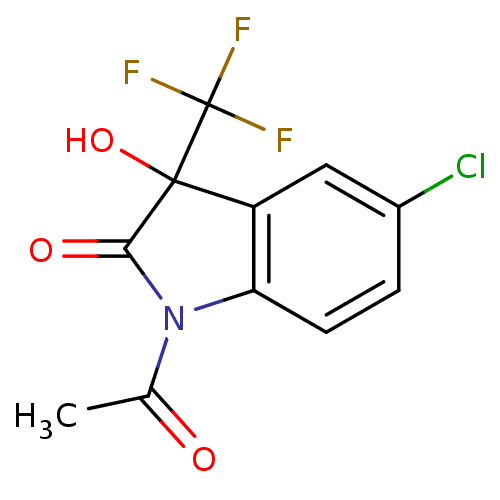
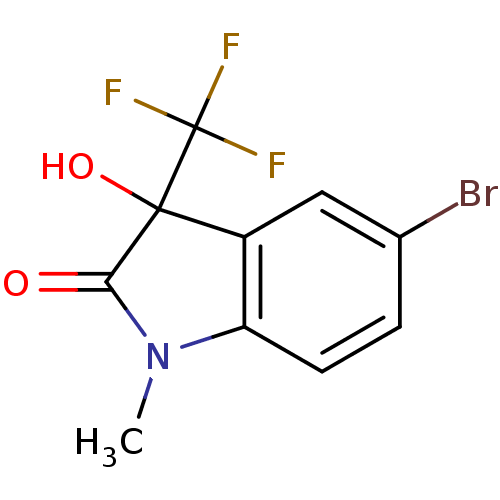
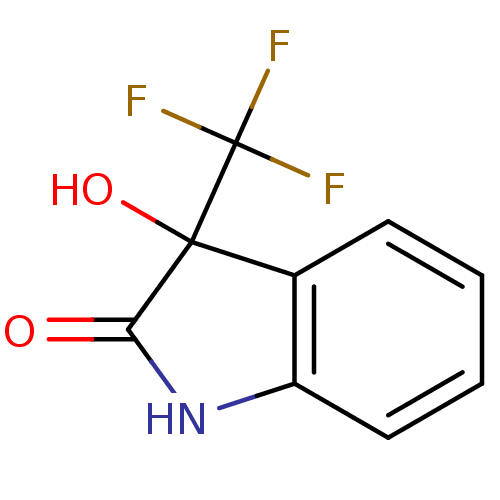
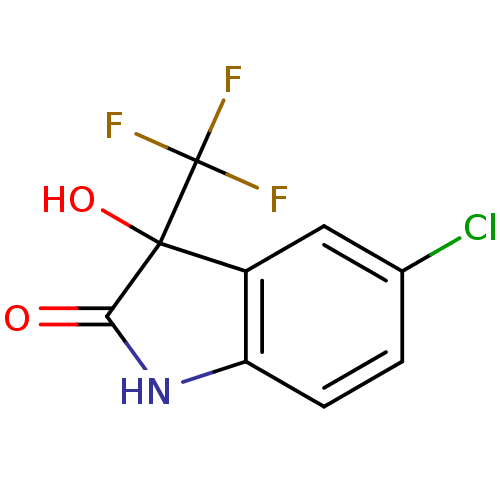
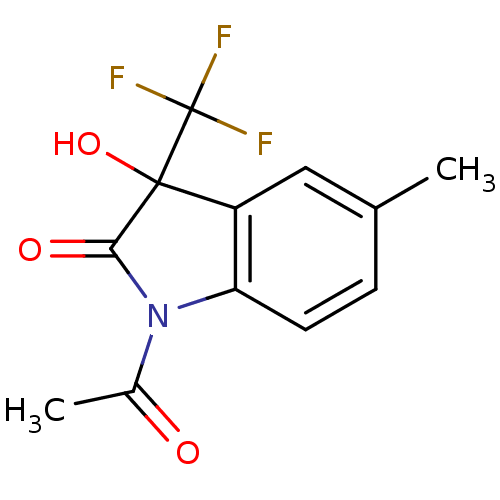
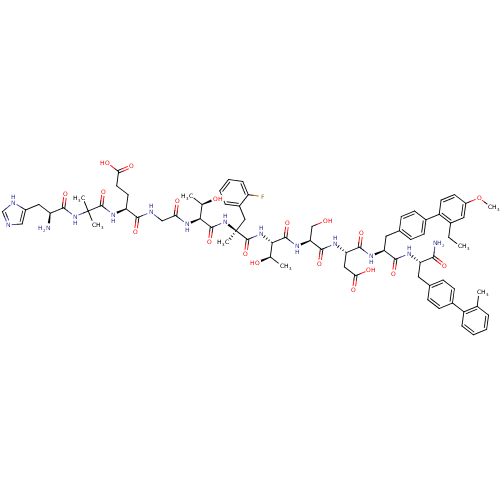
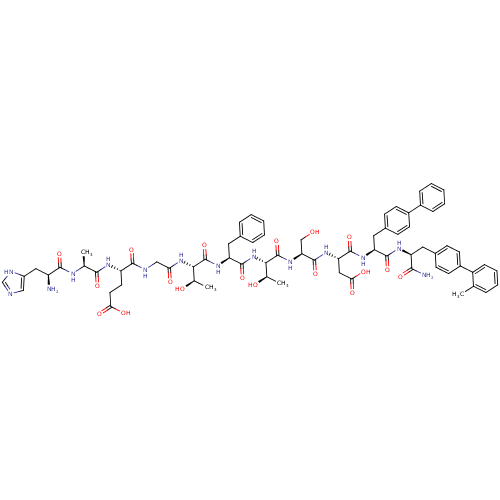
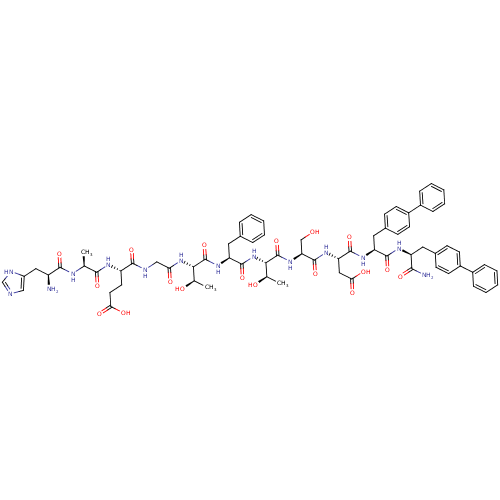
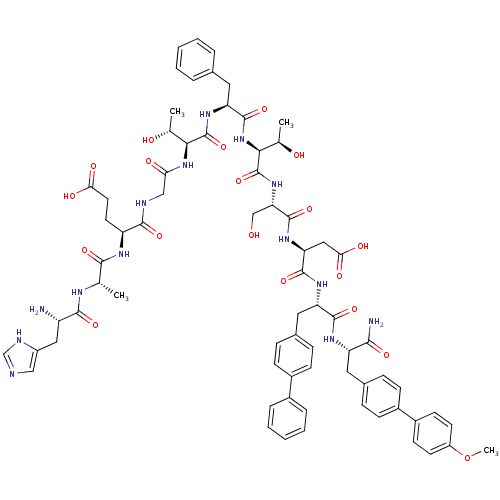
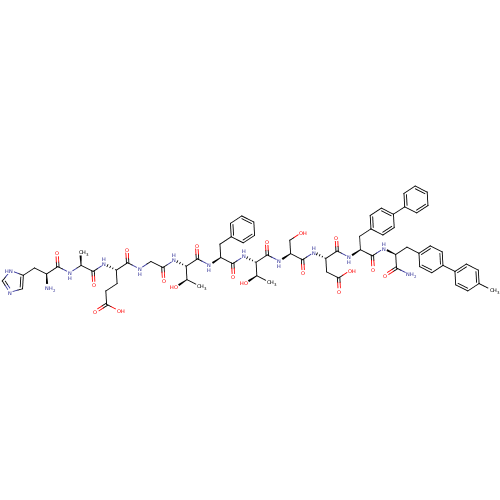
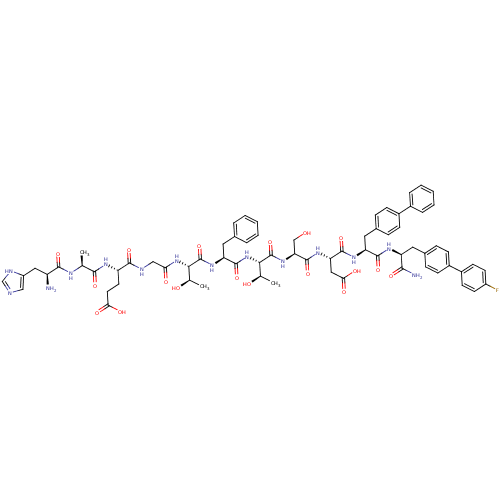
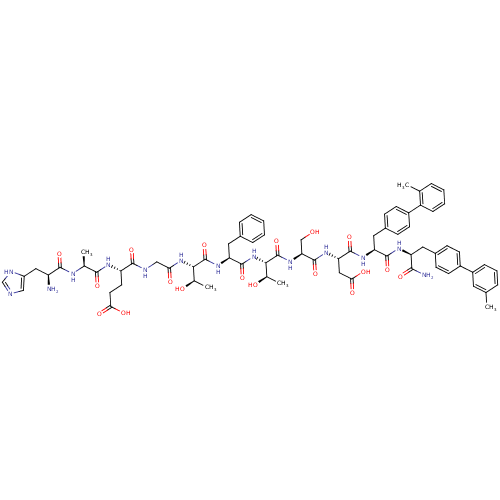
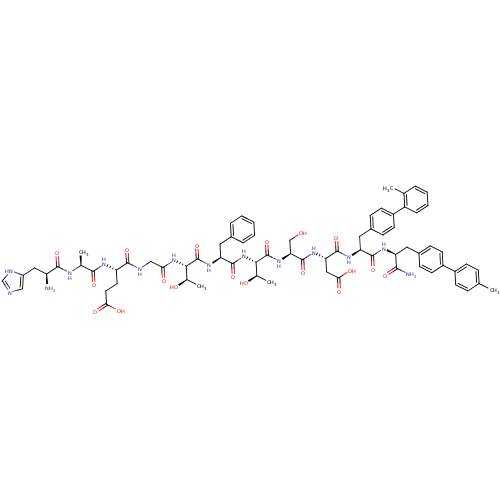
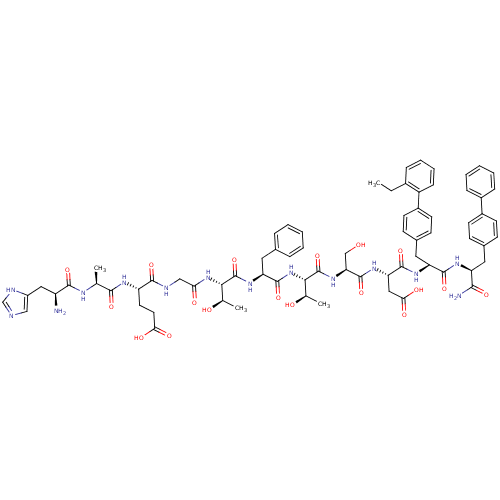
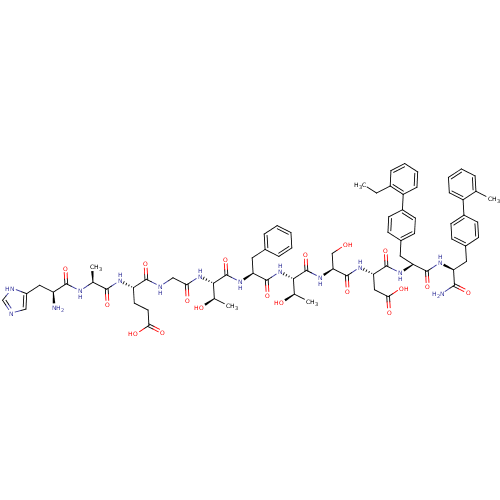
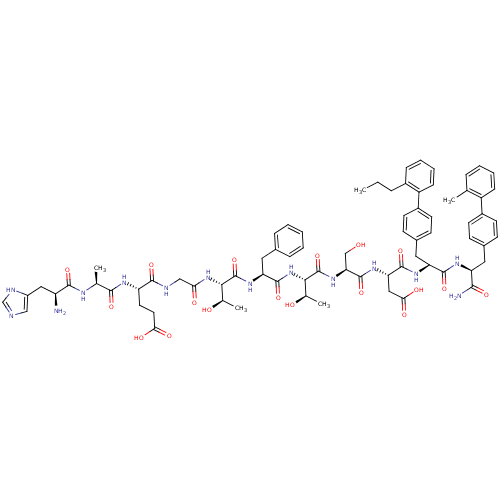
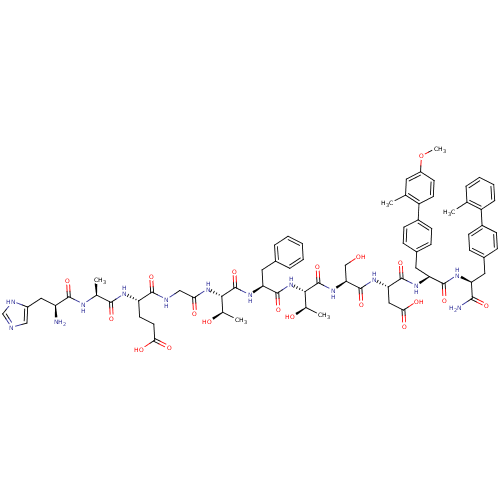
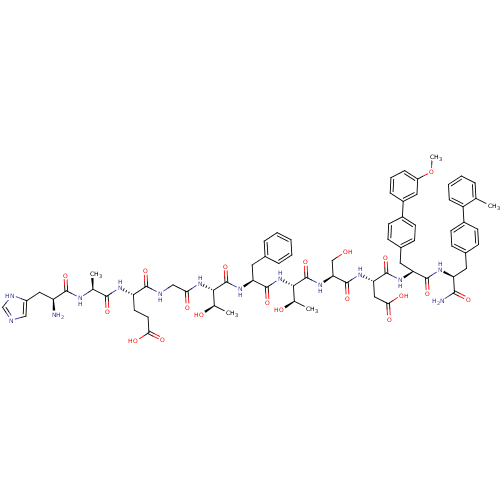
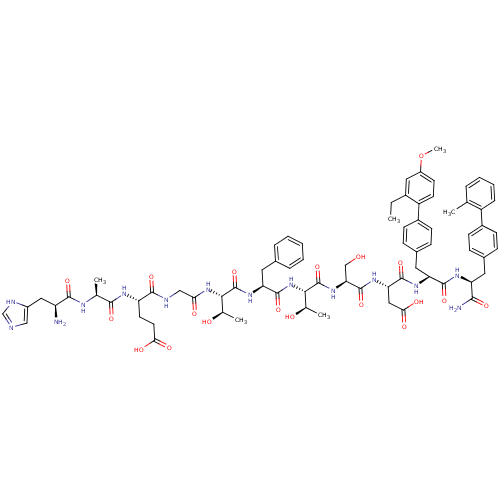
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
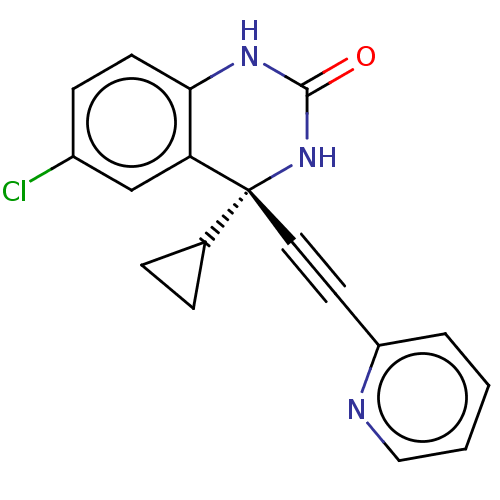
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
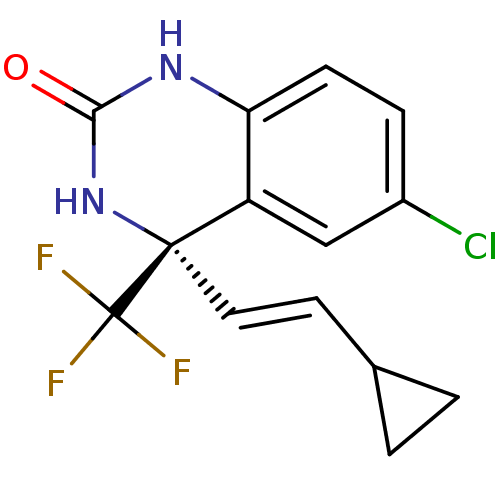
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
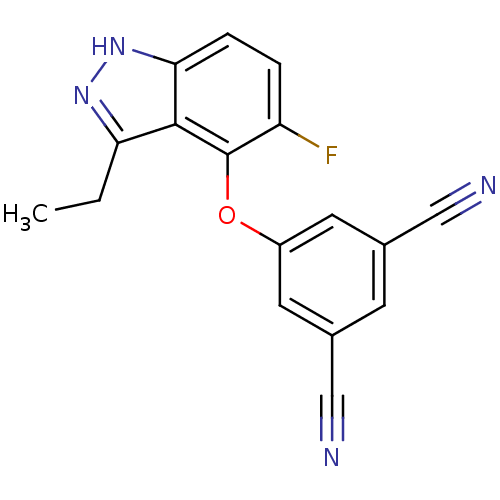
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 59nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.41E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.41E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.61E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.61E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 6.07E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 6.07E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 7.10E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 8.37E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 8.37E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetParathyroid hormone/parathyroid hormone-related peptide receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Agonist activity at PTHRMore data for this Ligand-Target Pair
TargetGastric inhibitory polypeptide receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Agonist activity at GIPRMore data for this Ligand-Target Pair
TargetParathyroid hormone/parathyroid hormone-related peptide receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Agonist activity at PTHRMore data for this Ligand-Target Pair
TargetGlucagon receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Agonist activity at glucagon receptorMore data for this Ligand-Target Pair
TargetGastric inhibitory polypeptide receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Agonist activity at GIPRMore data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 480nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 545nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 390nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 270nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 430nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 148nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 87nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 95nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 27nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 22nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 93nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 35nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 965nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 7nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 0.0340nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair
TargetGlucagon-like peptide 1 receptor(Homo sapiens (Human))
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Bristol-Myers Squibb Company Research& Development
Curated by ChEMBL
Affinity DataEC50: 2.40nMAssay Description:Agonist activity at human GLP1R expressed in CHO cells assessed as stimulation of intracellular [3H]cAMP accumulation after 30 mins by scintillation ...More data for this Ligand-Target Pair