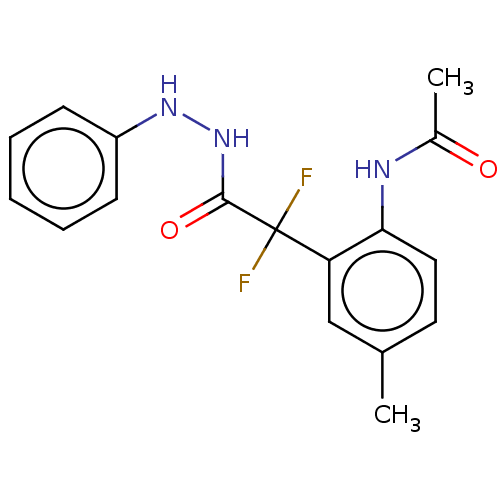
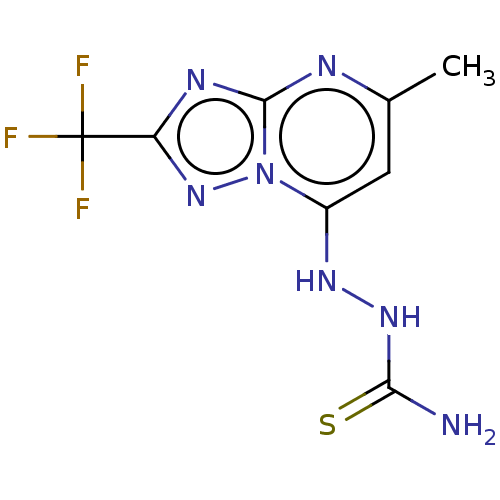
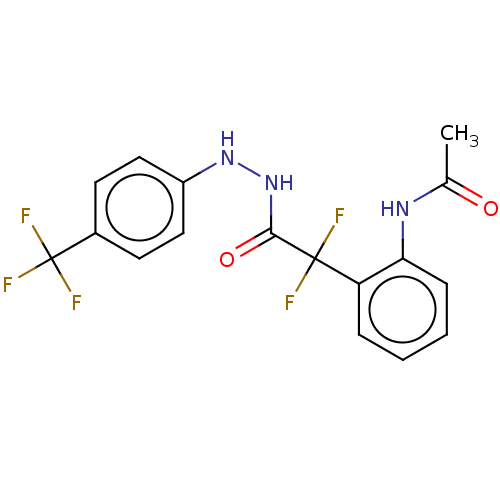
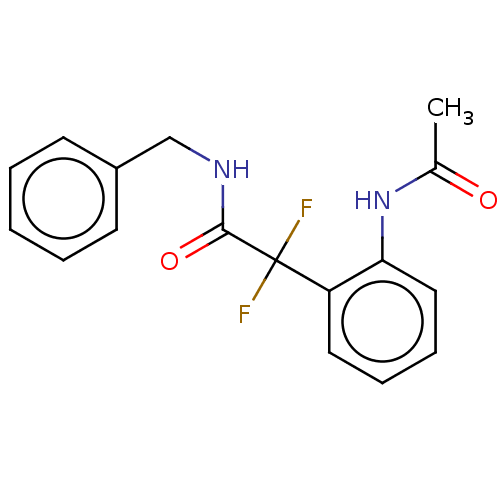
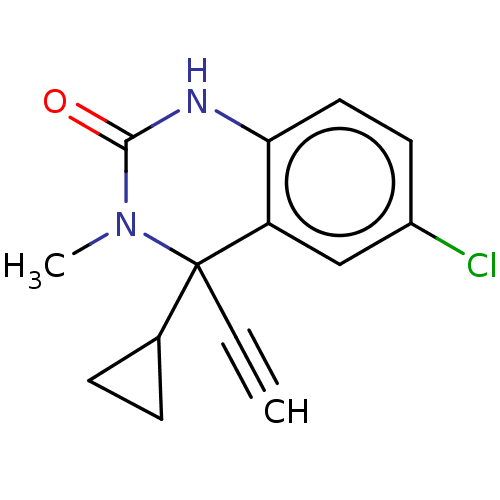
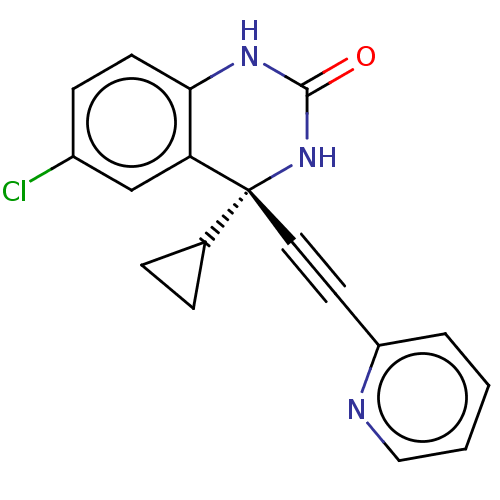
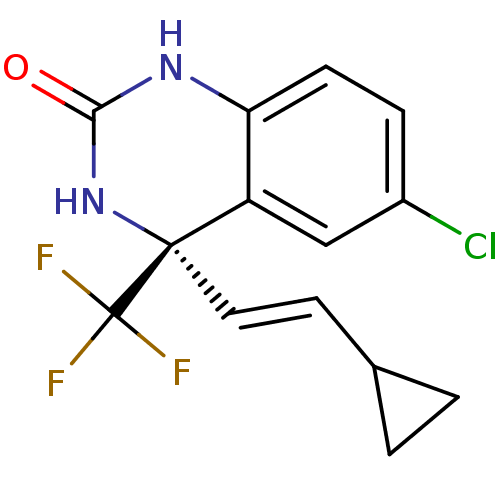
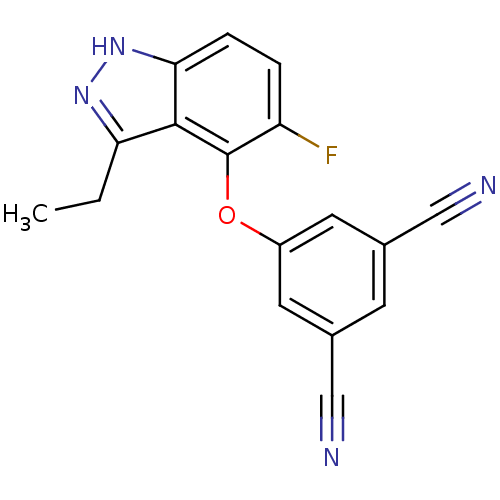
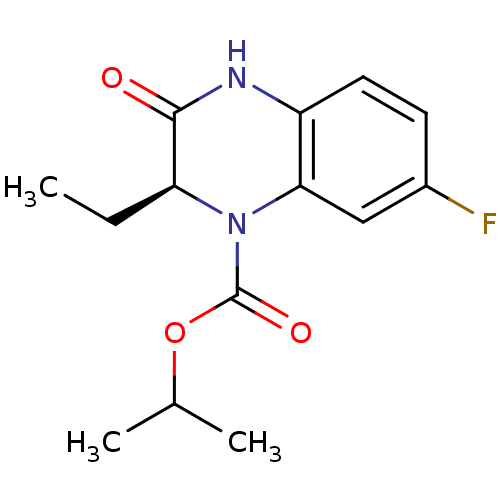
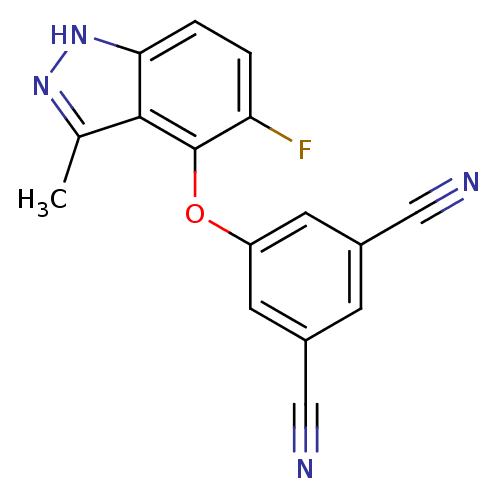
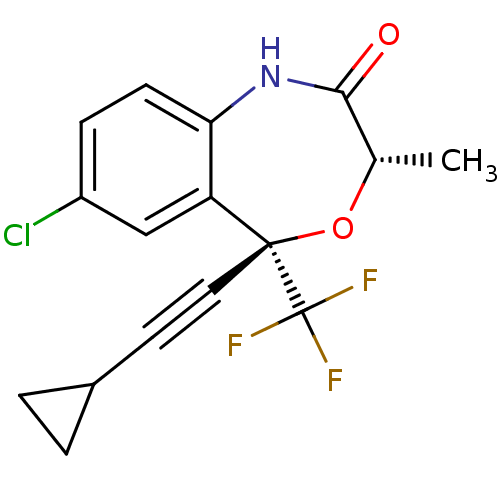
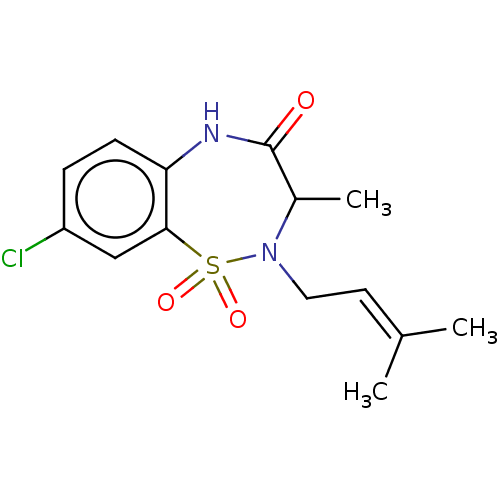
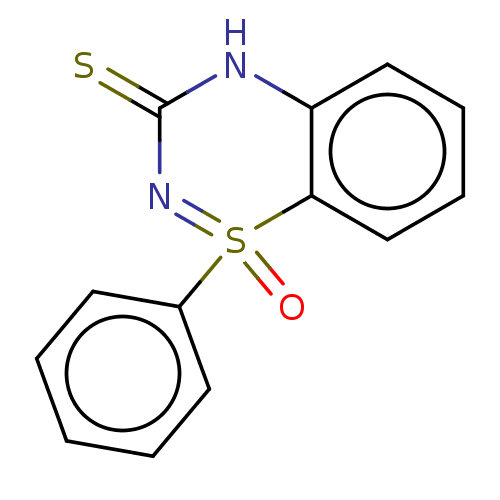
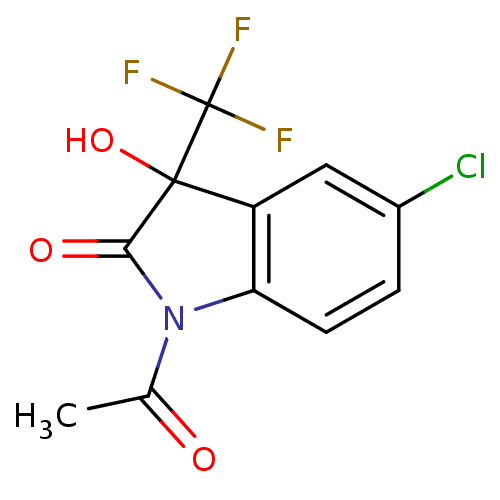
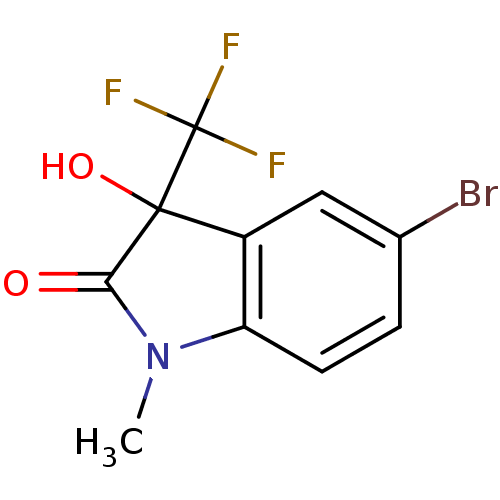
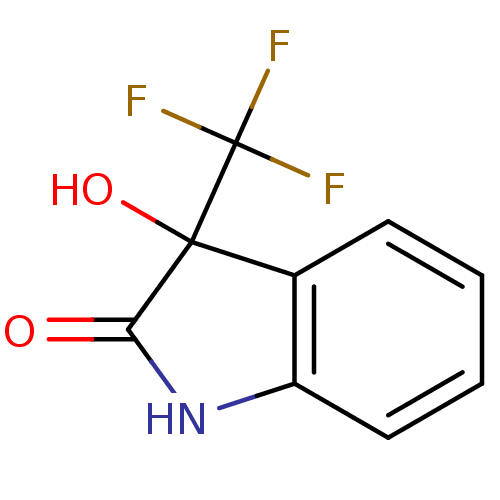
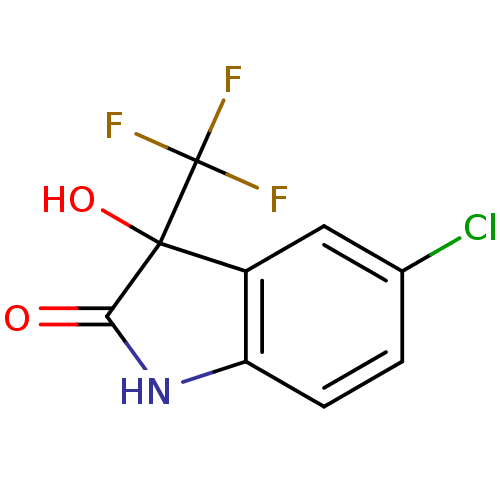
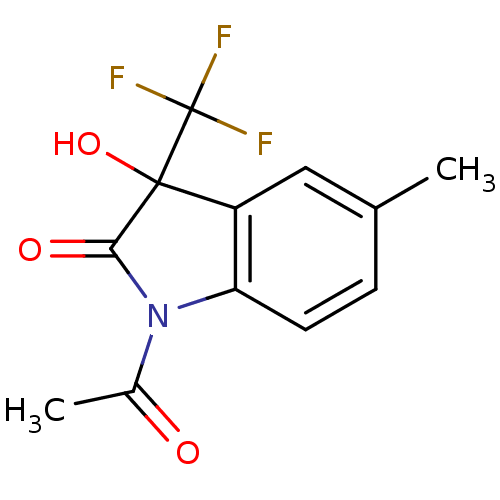
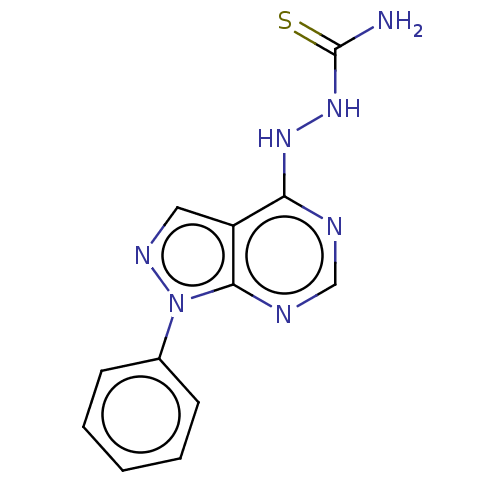
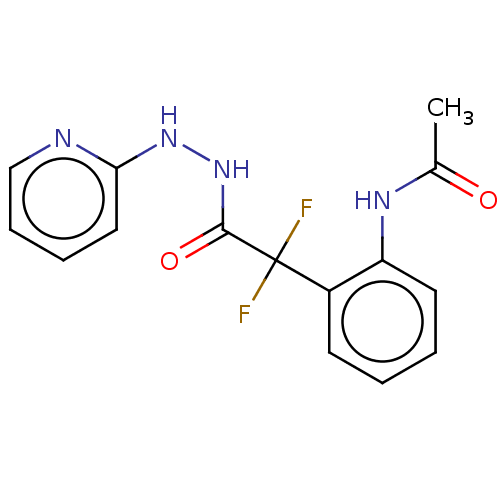
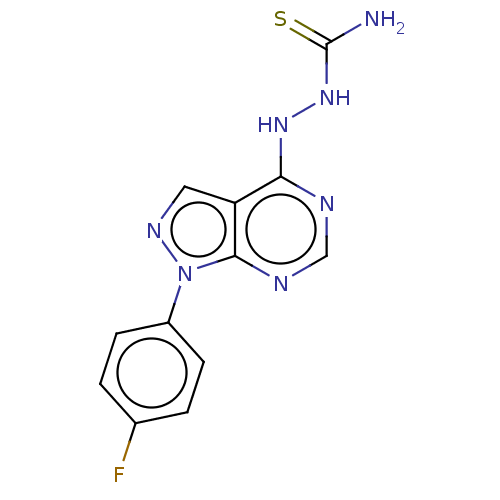
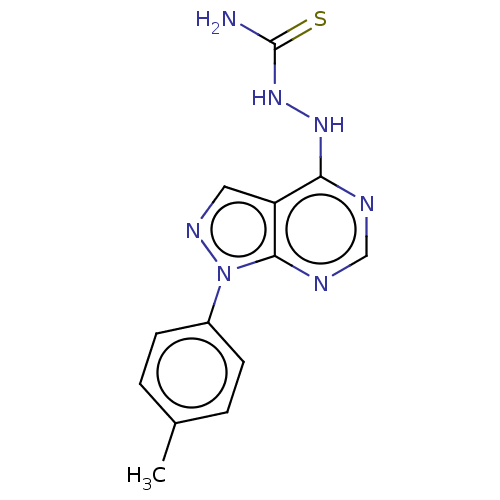
Affinity DataKi: 1.30E+3nMAssay Description:Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu...More data for this Ligand-Target Pair
Affinity DataKi: 5.10E+3nMAssay Description:Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu...More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 minsMore data for this Ligand-Target Pair
Affinity DataKi: 2.60E+4nMAssay Description:Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu...More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+4nMAssay Description:Competitive inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production by measu...More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 25nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 32nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 45nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 59nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 800nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 950nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.41E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.41E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.61E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 3.61E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 6.07E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 6.07E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 7.10E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 7.10E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 8.37E+3nMAssay Description:Biological assay using HIV-1 reverse transcriptase.More data for this Ligand-Target Pair
TargetReverse transcriptase(Human immunodeficiency virus 1)
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Instituto de Tecnologia em F£rmacos
Curated by ChEMBL
Affinity DataIC50: 8.37E+3nMAssay Description:Inhibition of HIV1 reverse transcriptaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.65E+4nMAssay Description:Non-competitive inhibition of Leishmania amazonensis arginase using L-arginine as substrate incubated for 15 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.65E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli assessed as reduction in urea production using L-arginine as ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant Leishmania amazonensis arginase expressed in Escherichia coli using L-arginine as substrateMore data for this Ligand-Target Pair