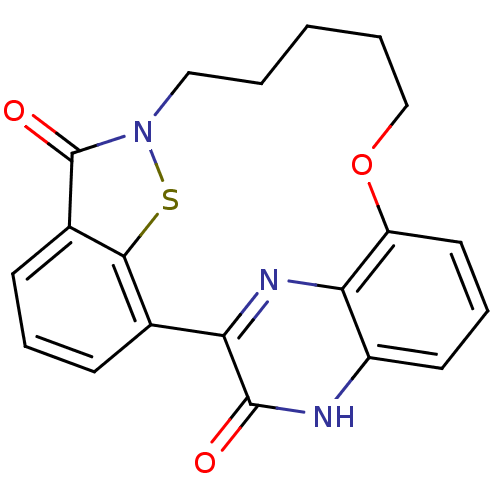
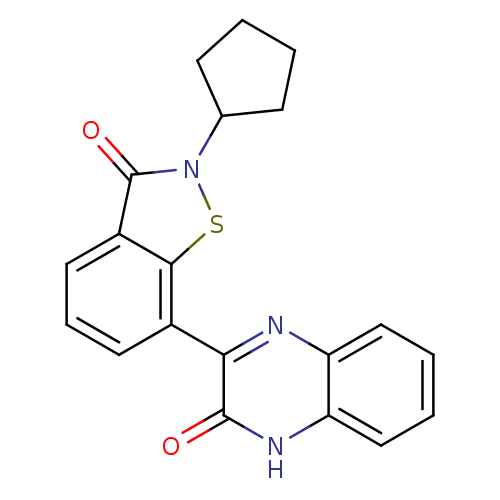
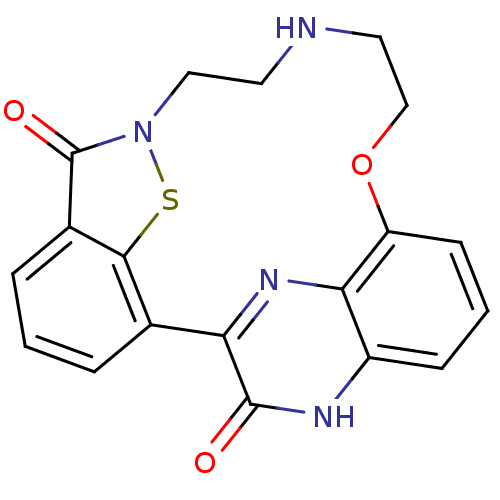
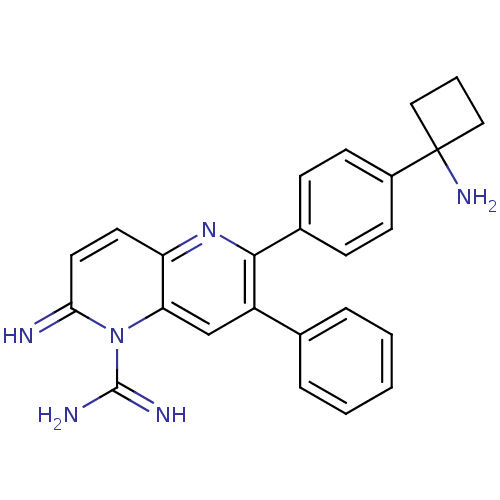
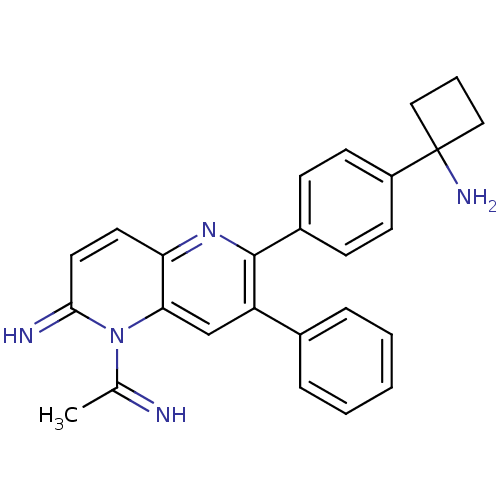
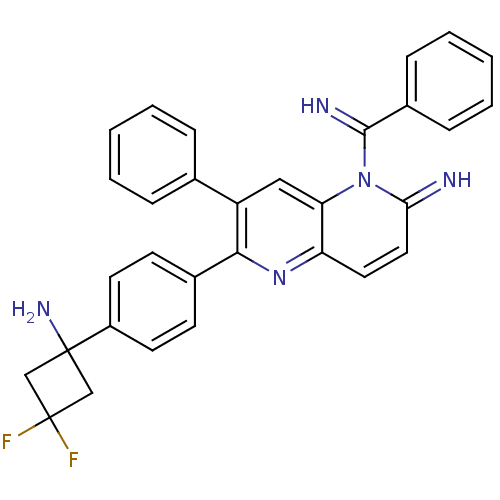
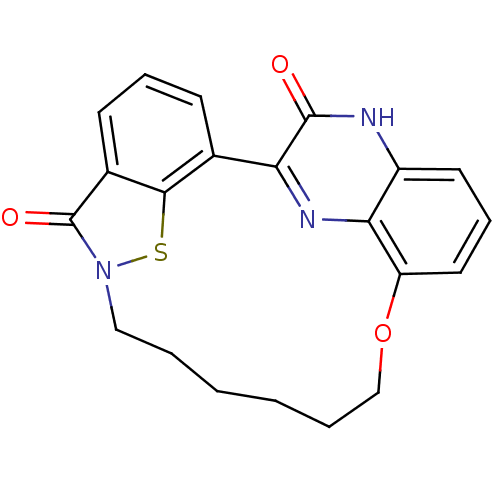
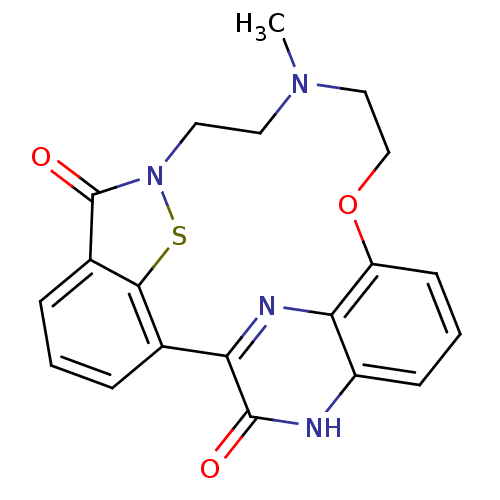
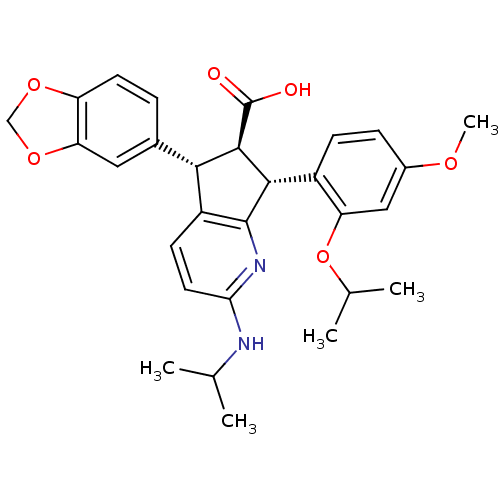
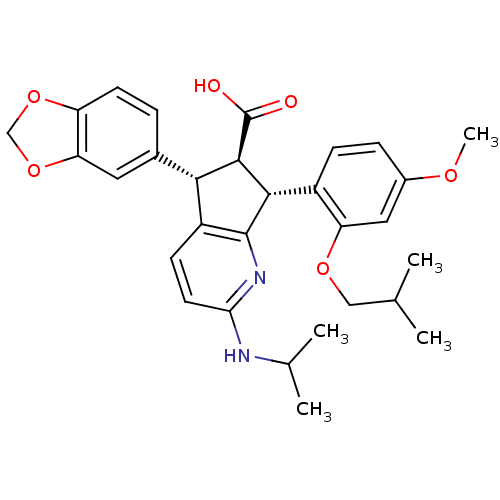
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
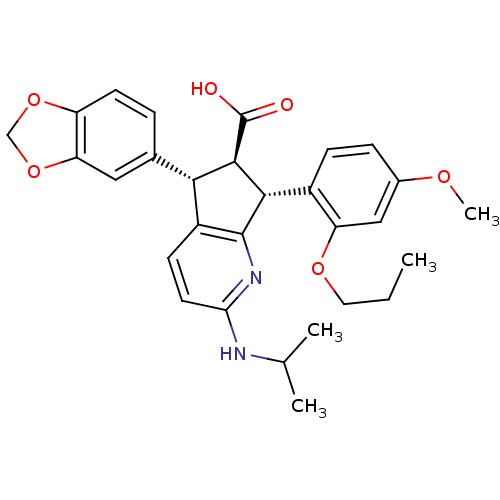
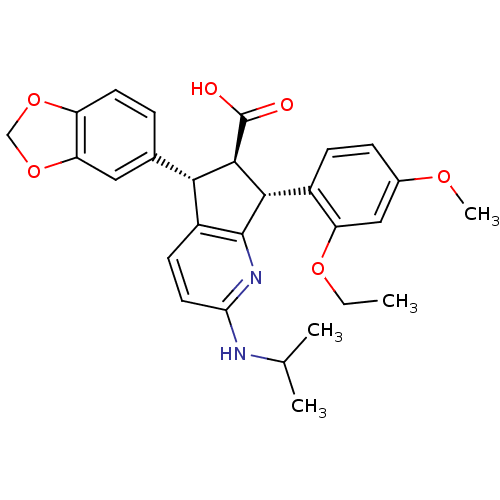
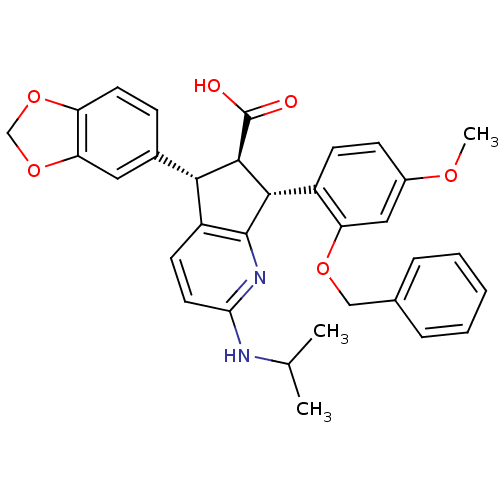
Affinity DataIC50: 0.0110nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair
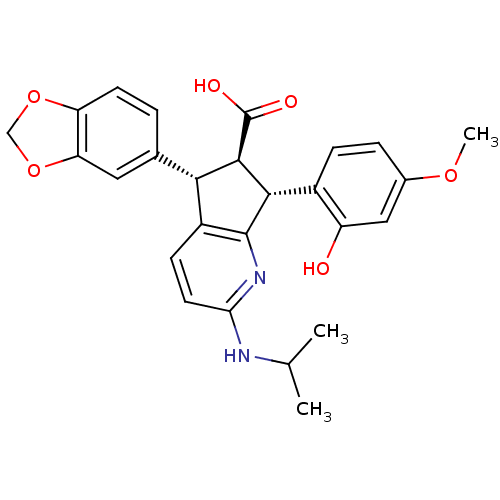
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0110nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0110nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0110nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0140nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0220nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0240nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0240nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0270nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0310nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0470nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0480nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0480nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0570nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0570nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0590nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0590nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0620nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.0650nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.110nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.220nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
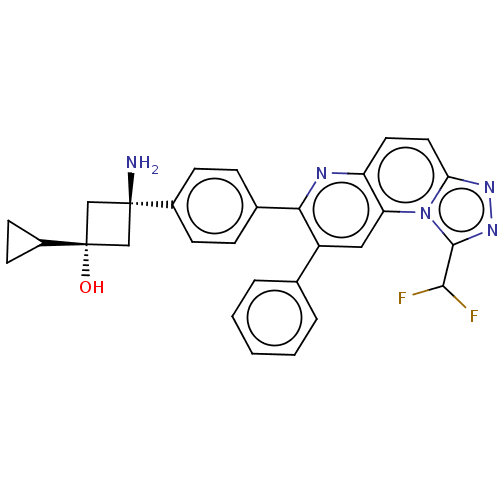
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 0.340nMAssay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
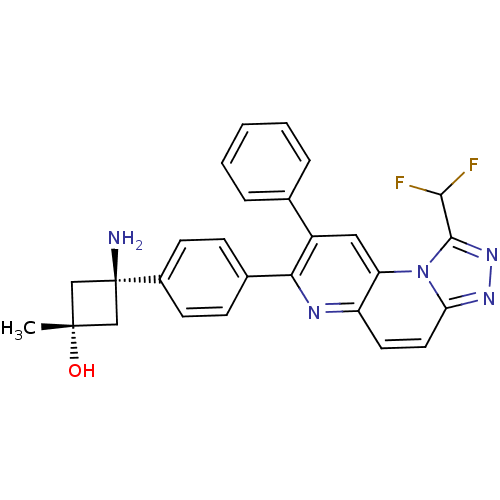
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 0.380nMpH: 7.5Assay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.410nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 0.700nMAssay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.740nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin-1 receptor(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.860nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C](Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 1.60nMAssay Description:In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d...More data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 1.70nMpH: 7.5Assay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 2.30nMpH: 7.5Assay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1 [L188C](Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 2.30nMAssay Description:In vitro kinase assays using synthetic peptides and purified enzymes were incubated at 30°C for 45 min in buffer that contained 50 uM ATP, and d...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetEndothelin receptor type B(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetEndothelin receptor type B(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 6.10nMpH: 7.5Assay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 8.20nMpH: 7.5Assay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Homo sapiens (Human))
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Merck Sharp & Dohme Corp.; MSD K.K.
US Patent
Affinity DataIC50: 10nMpH: 7.5Assay Description:Activated Akt isoforms and pleckstrin homology domain deletion constructs were assayed utilizing a GSK-derived biotinylated peptide substrate. The e...More data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetCyclin-dependent kinase 6(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair

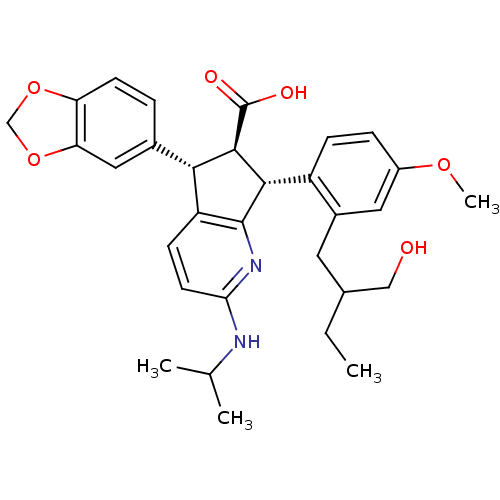
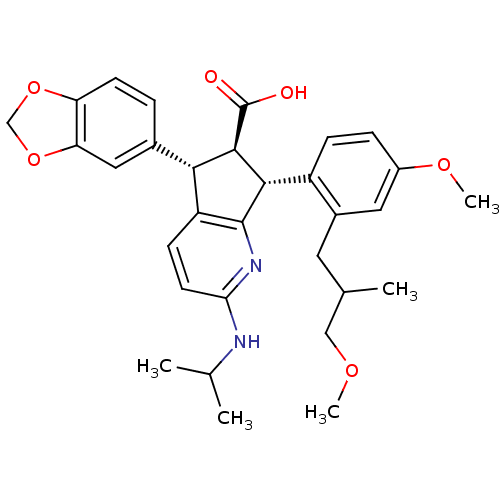

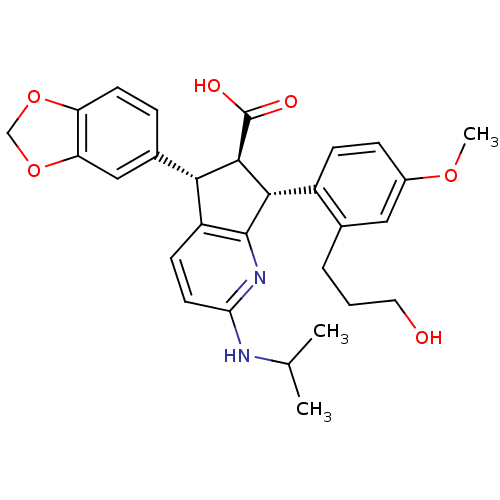
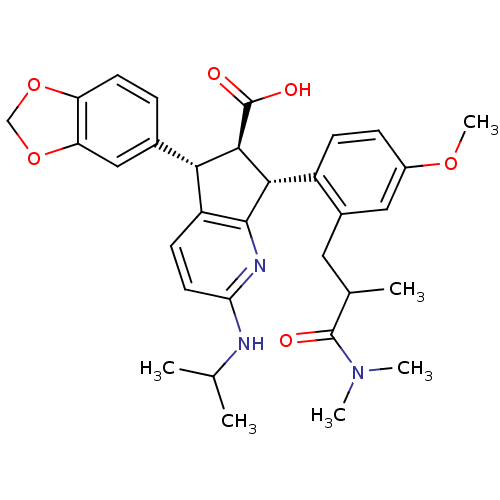
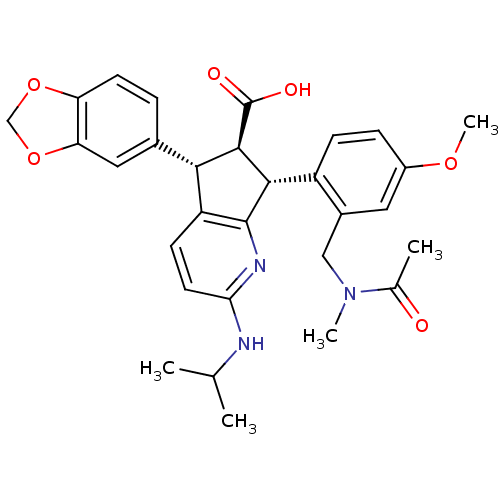
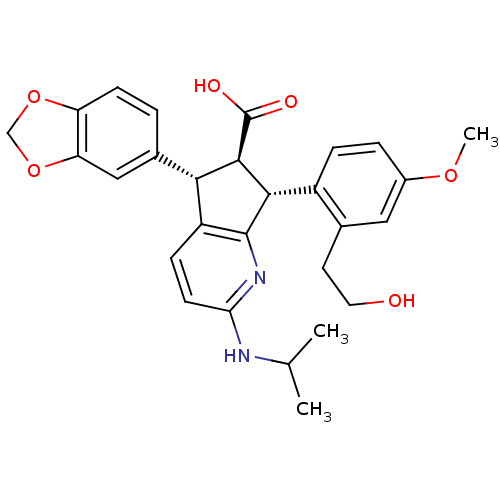
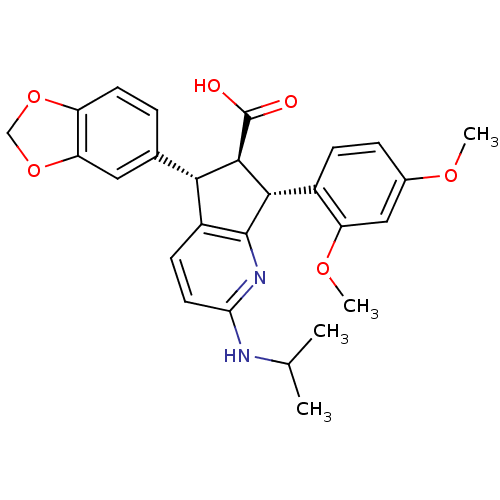
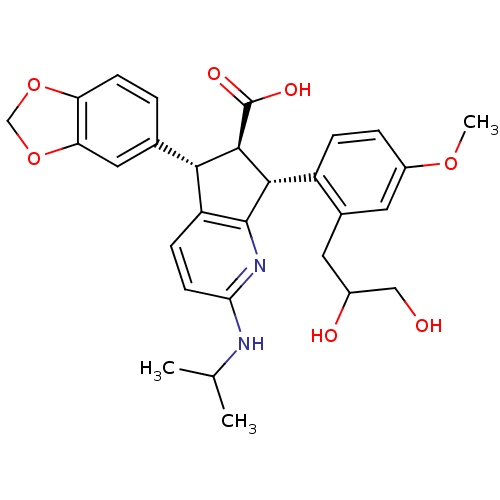
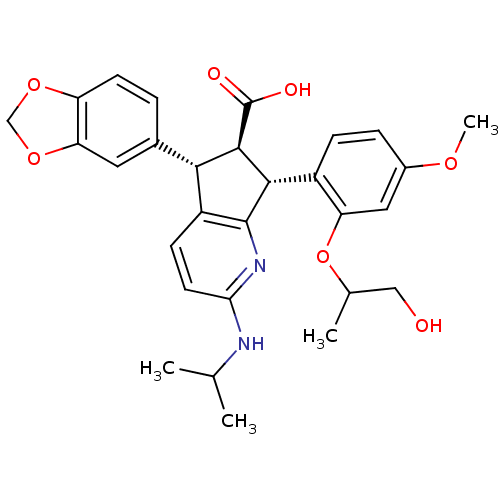
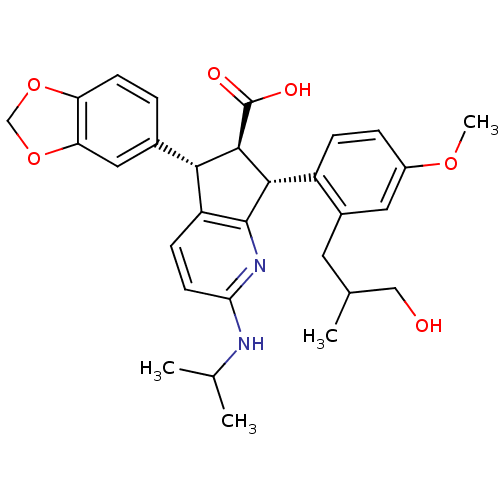
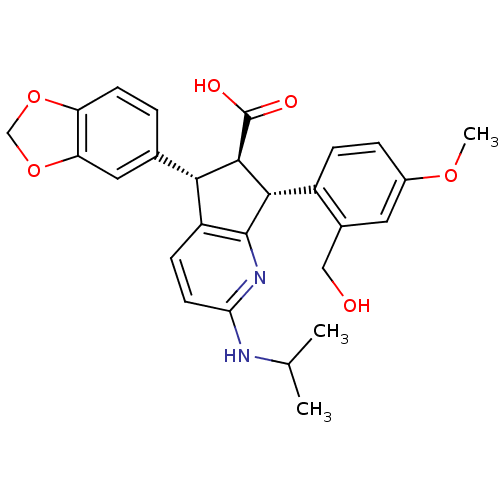
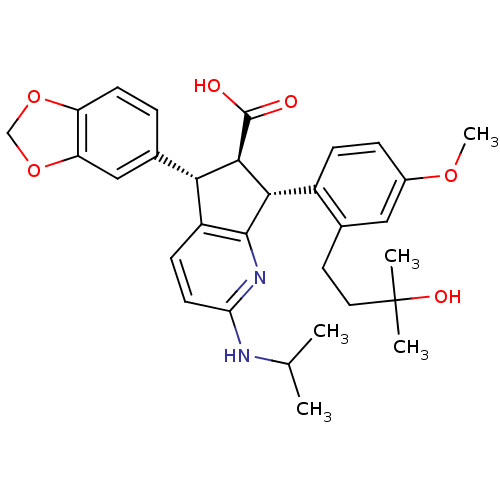

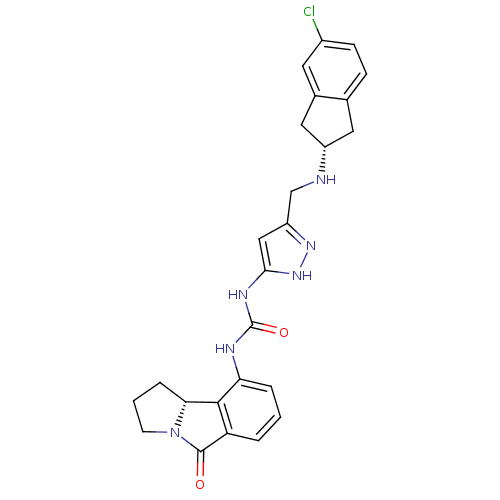
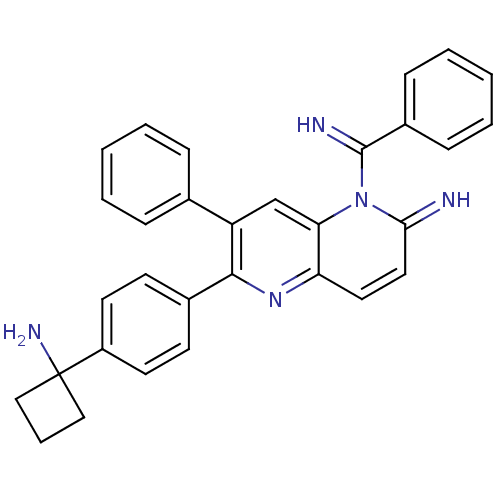
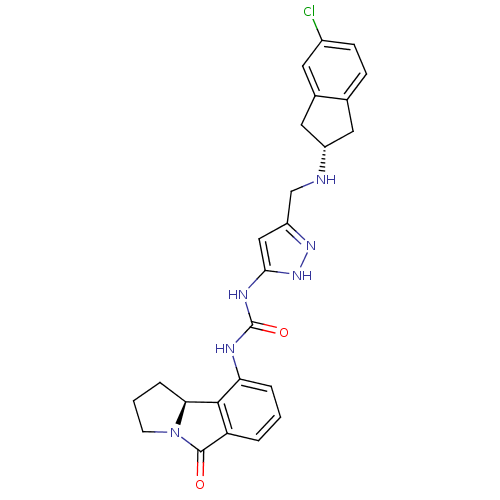

 3D Structure (crystal)
3D Structure (crystal)