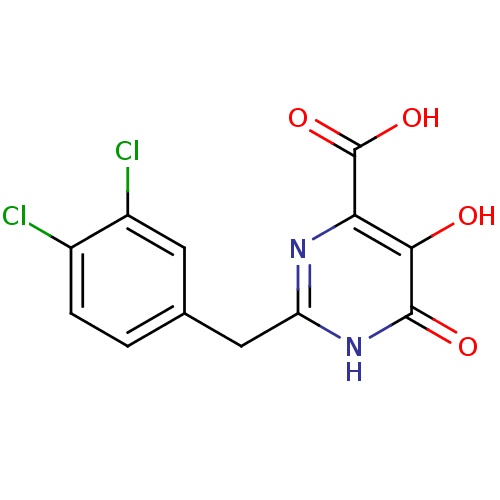
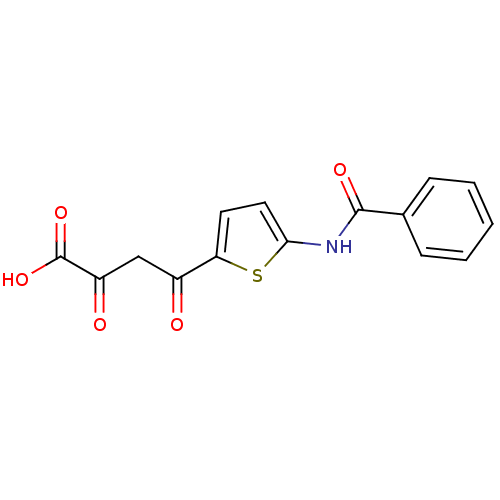
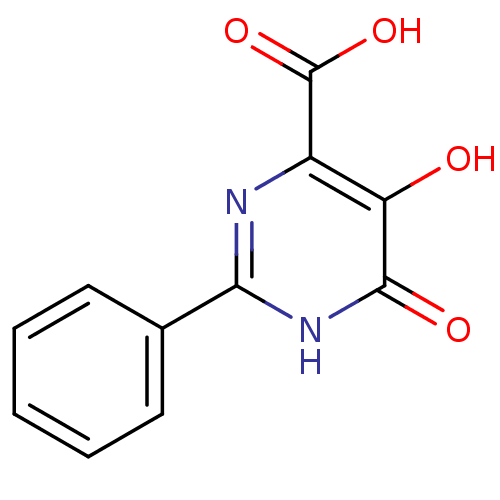
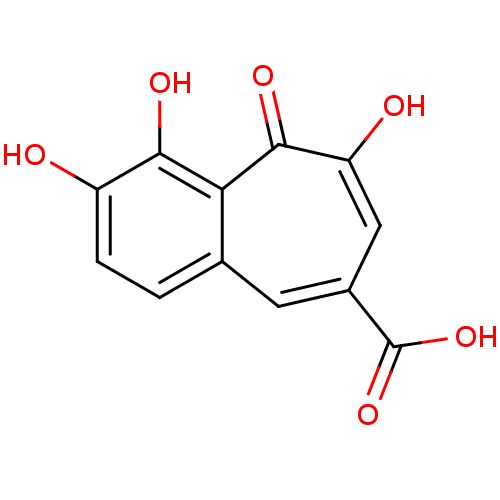
Affinity DataIC50: 50nMAssay Description:Inhibition of porcine liver carboxylesterase using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 135nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 175nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of recombinant CatA using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 660nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.15E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human leukocyte elastase using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 9.85E+3nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant CatA using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.32E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 4.85E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+4nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Enzyme activity was measuring substrate hydrolysis of RNase H. The intact substrate has a low background fluorescent signal and provides up to 50-fol...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+5nMAssay Description:Inhibition of porcine liver carboxylesterase using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of recombinant CatA using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of human leukocyte elastase using [14C]GS7340 substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of porcine liver carboxylesterase using [14C]GS7340 substrateMore data for this Ligand-Target Pair


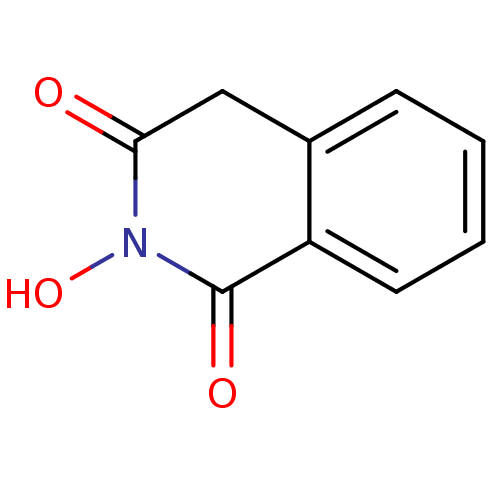
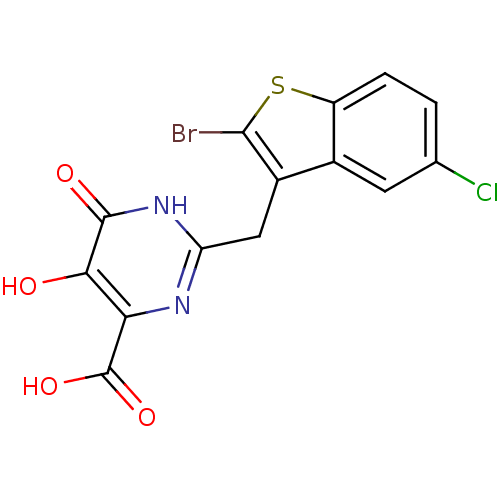
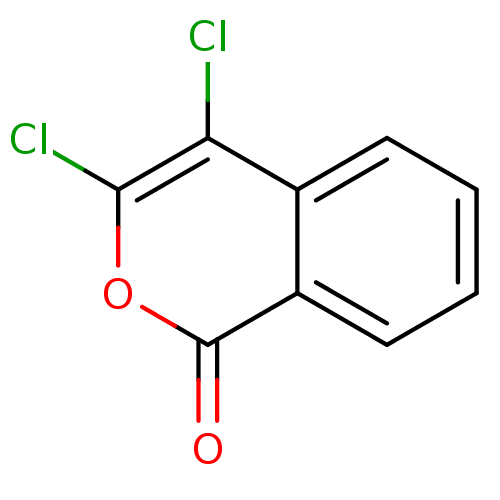
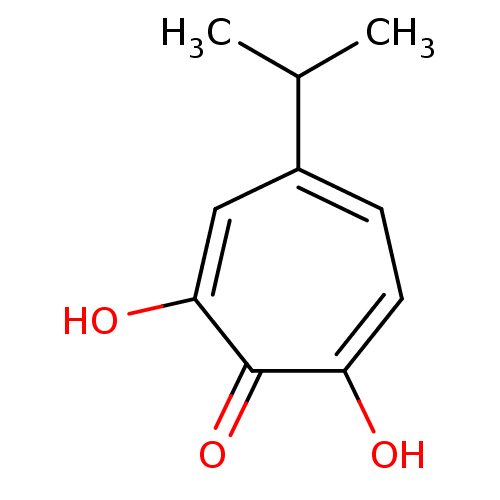
 3D Structure (crystal)
3D Structure (crystal)