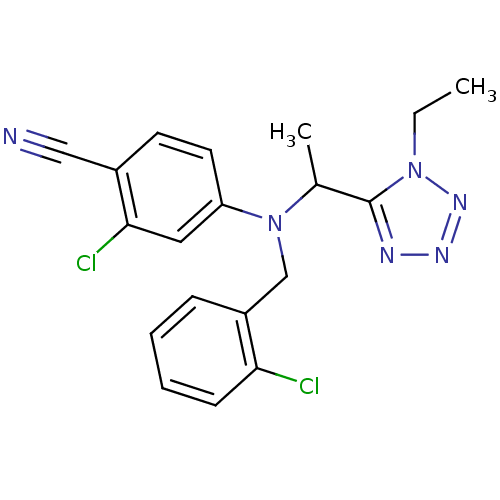
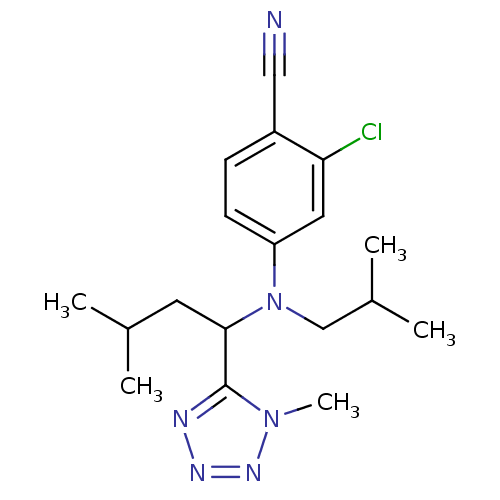
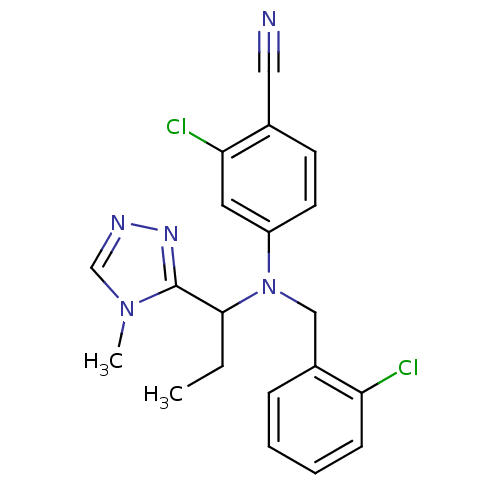
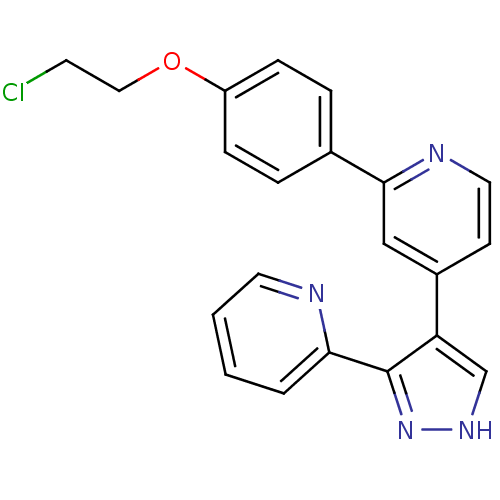
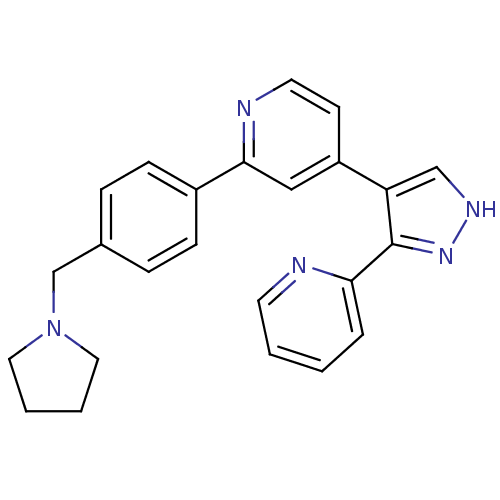
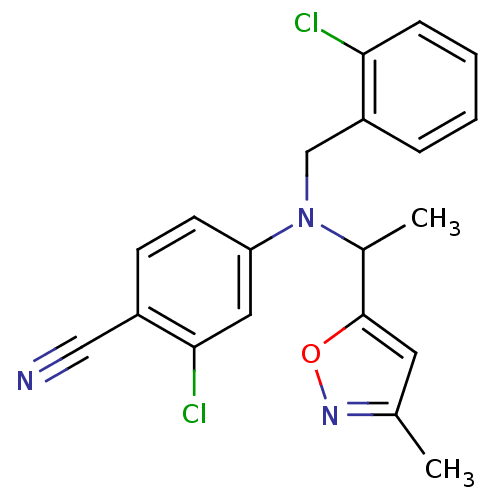
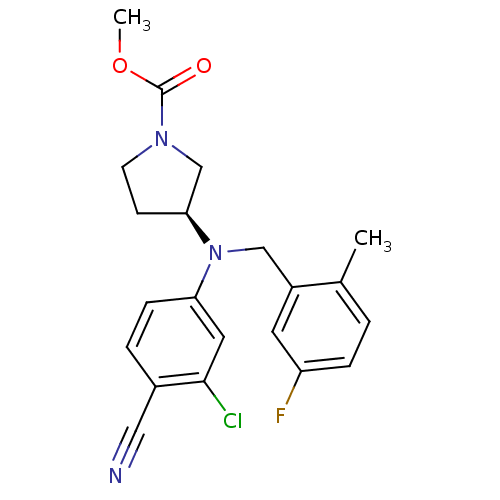
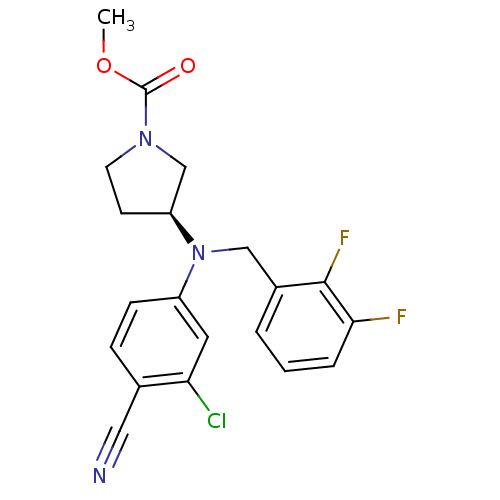
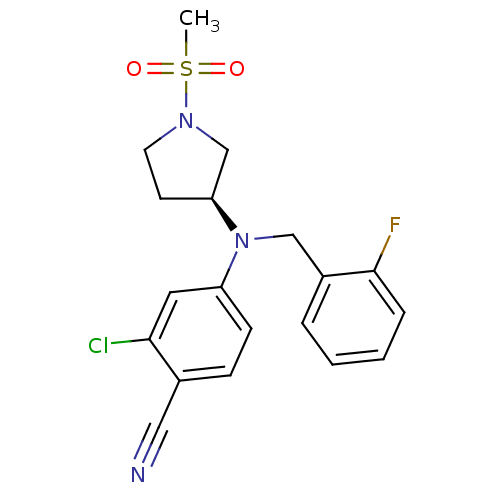
Affinity DataKi: 3.16nMAssay Description:Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ...More data for this Ligand-Target Pair
Affinity DataKi: 3.16nMAssay Description:Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs...More data for this Ligand-Target Pair
Affinity DataKi: 5.01nMAssay Description:Binding affinity to human EP3More data for this Ligand-Target Pair
Affinity DataKi: 6.31nMAssay Description:Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ...More data for this Ligand-Target Pair
Affinity DataKi: 7.94nMAssay Description:Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ...More data for this Ligand-Target Pair
Affinity DataKi: 12.6nMAssay Description:Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs...More data for this Ligand-Target Pair
Affinity DataKi: 19.9nMAssay Description:Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ...More data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ...More data for this Ligand-Target Pair
Affinity DataKi: 316nMAssay Description:Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs...More data for this Ligand-Target Pair
Affinity DataKi: >5.01E+3nMAssay Description:Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataKi: >5.01E+4nMAssay Description:Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity to PR by fluorescence polarization based competition binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:Binding affinity to PR by fluorescence polarization based competition binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Displacement of rhodamine green fluorescently labeled ATP from recombinant GST-ALK5 by FP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Sgk1(Homo sapiens (Human))
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of SGK1 by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to PR by fluorescence polarization based competition binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Displacement of rhodamine green fluorescently labeled ATP from recombinant GST-ALK5 by FP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to PR by fluorescence polarization based competition binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Displacement of rhodamine green fluorescently labeled ATP from recombinant GST-ALK5 by FP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Displacement of rhodamine green fluorescently labeled ATP from recombinant GST-ALK5 by FP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Displacement of rhodamine green fluorescently labeled ATP from recombinant GST-ALK5 by FP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Binding affinity to progesterone receptor ligand binding domain by fluorimetric assayMore data for this Ligand-Target Pair



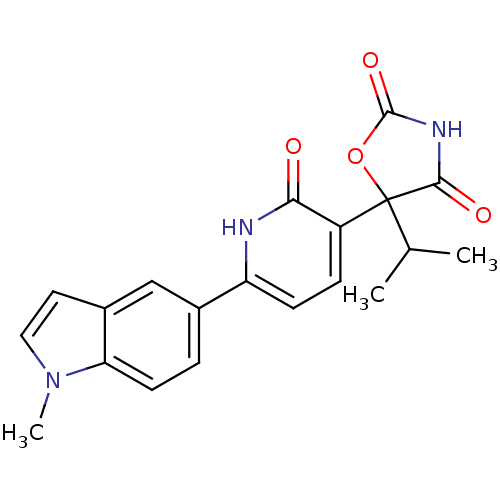

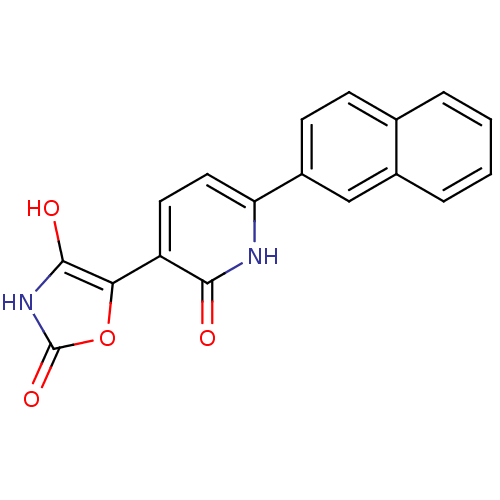




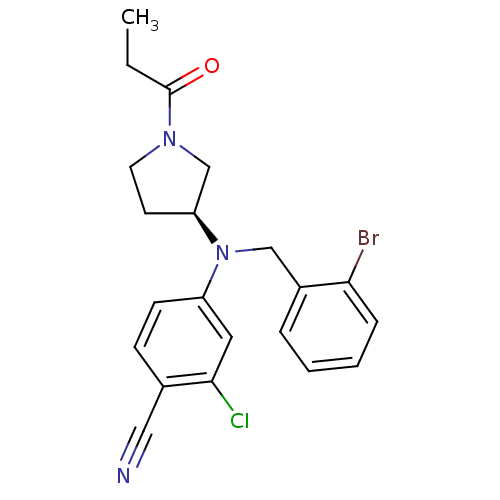





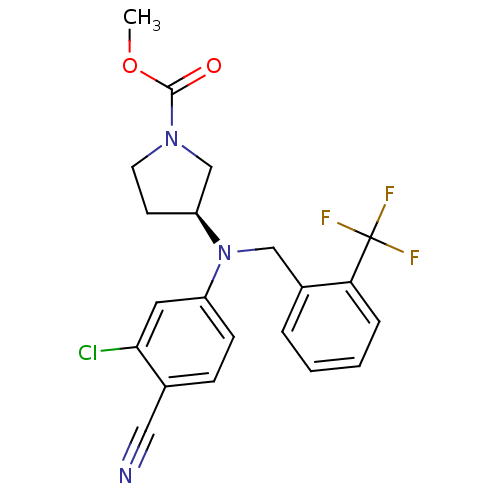

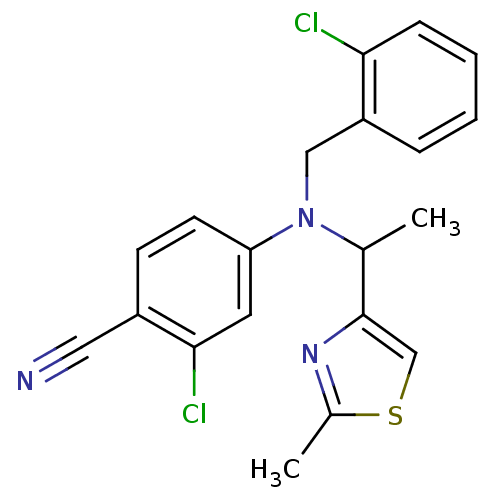


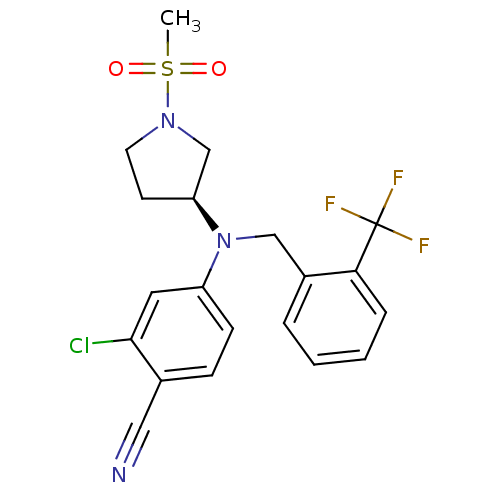
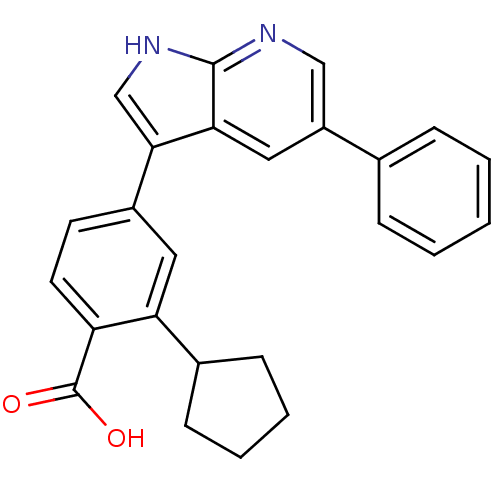

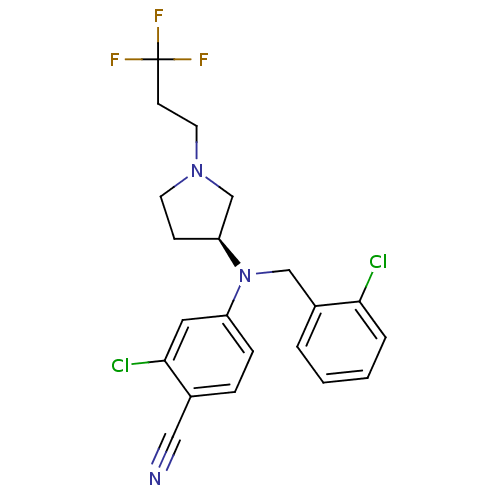

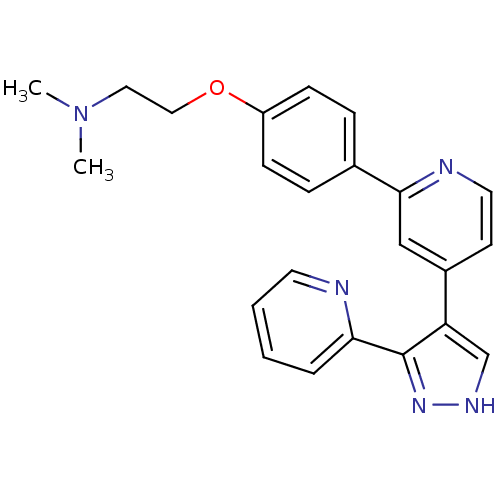
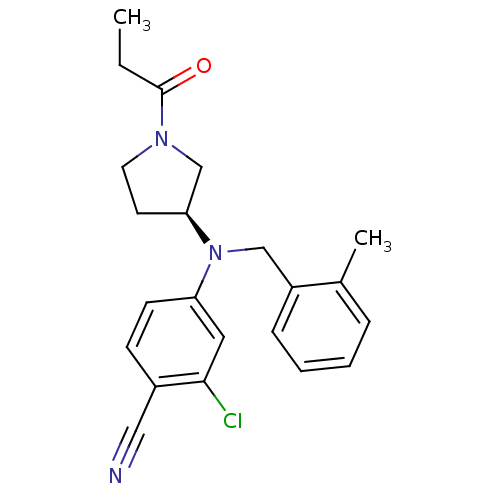

 3D Structure (crystal)
3D Structure (crystal)