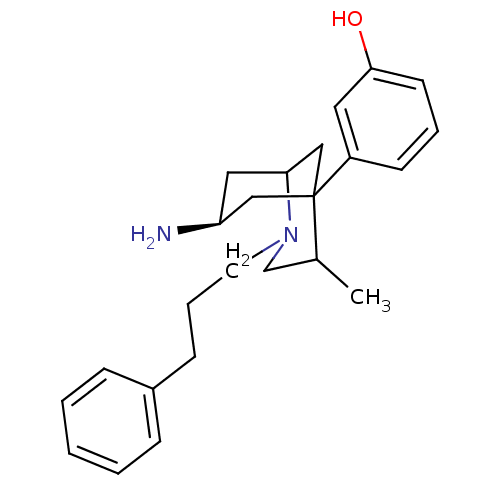
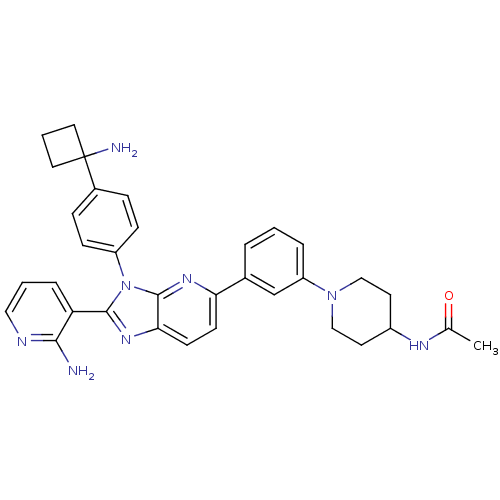
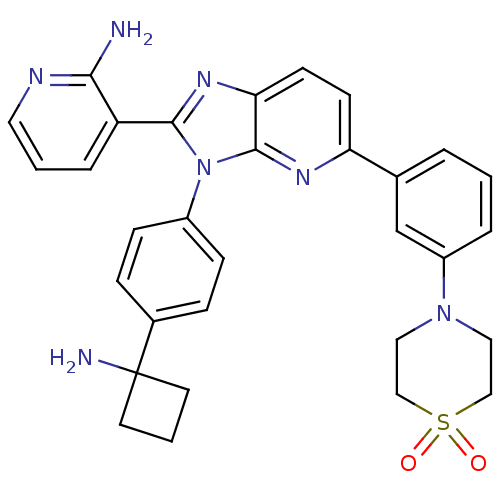
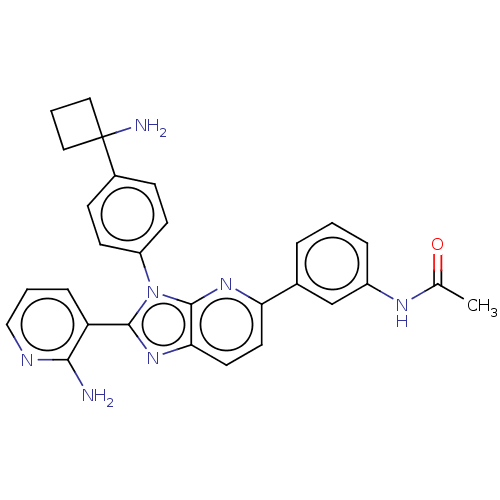
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 1.09nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
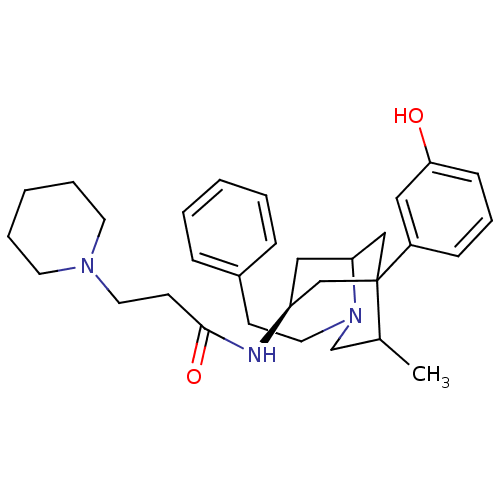
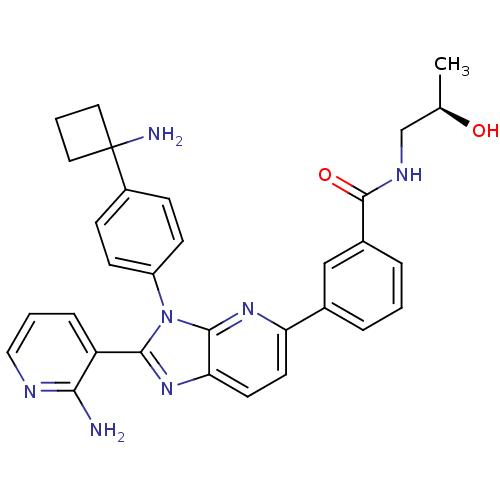
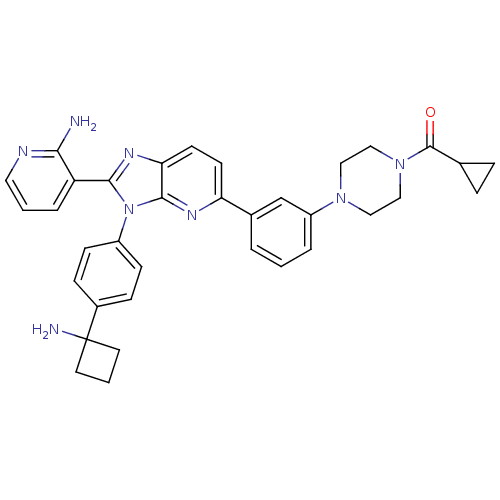
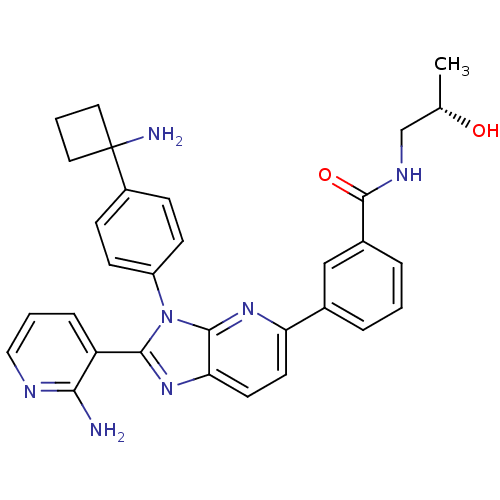
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 4.30nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
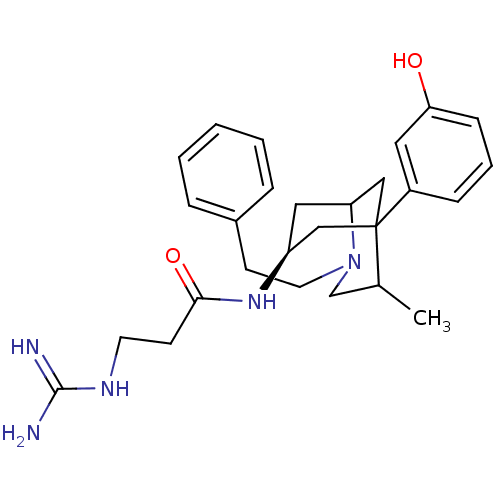
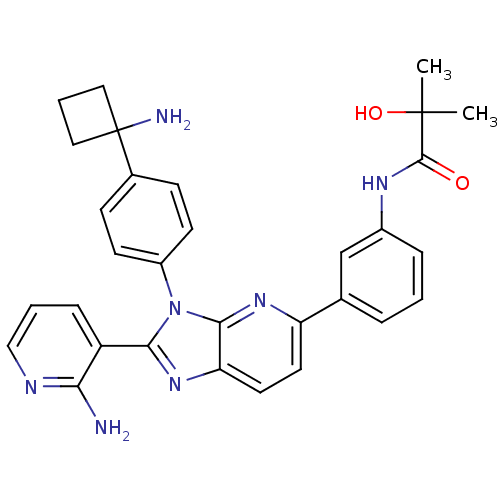
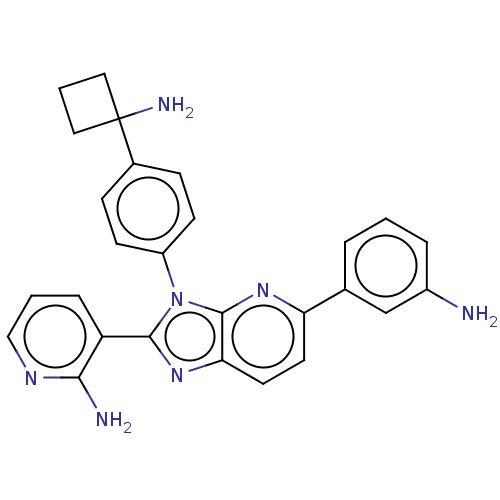
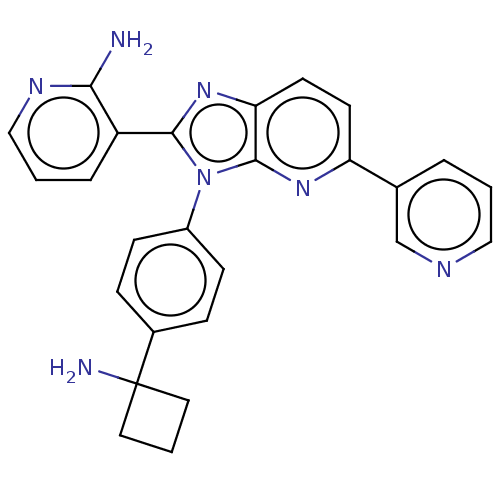
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
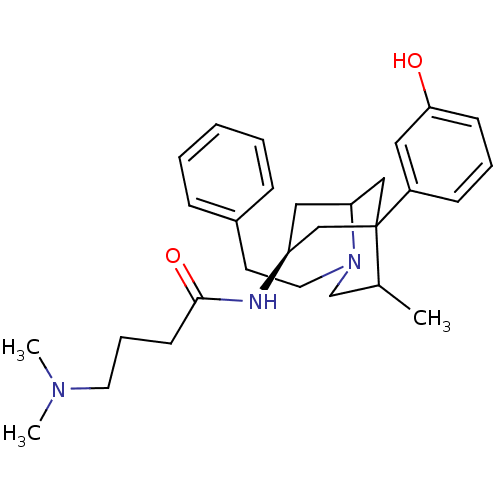
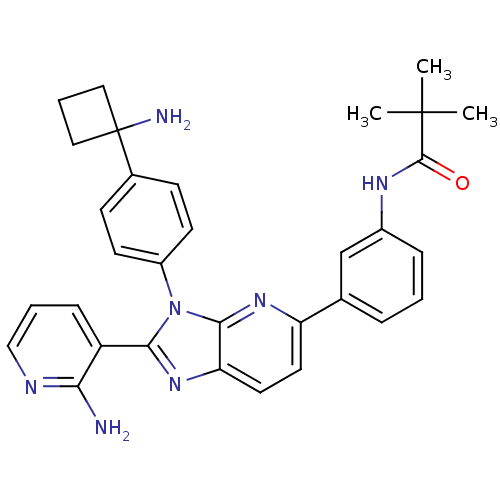
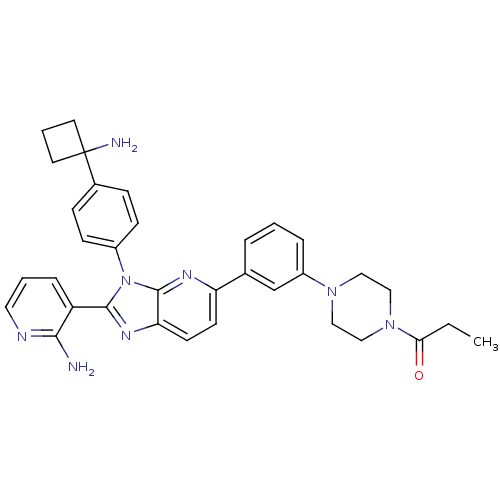
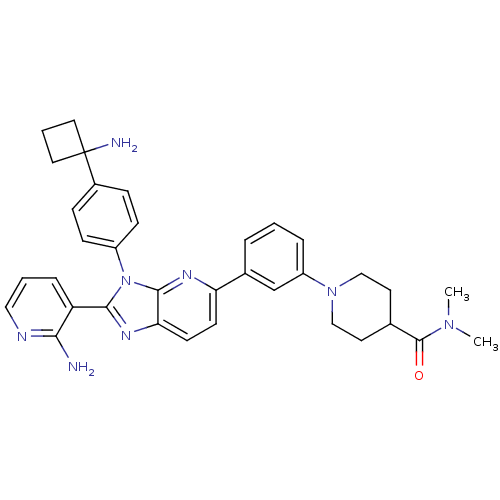
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 13.2nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 14.7nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 23.3nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 56.5nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
Affinity DataKi: 57nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 86nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
Affinity DataKi: 87nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 122nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
Affinity DataKi: 147nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
Affinity DataKi: 207nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 246nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
Affinity DataKi: 775nMAssay Description:Ability to displace [3H]DAMGO from Opioid receptor mu 1 of rat brainMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 893nMAssay Description:Ability to displace [3H]U-69593 from Opioid receptor kappa 1 of guinea pig brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 1.46E+3nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 1.74E+3nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: 2.18E+3nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Research Triangle Institute
Curated by ChEMBL
Research Triangle Institute
Curated by ChEMBL
Affinity DataKi: >3.40E+3nMAssay Description:Ability to displace [3H]DADLE from Opioid receptor delta 1 of rat brainMore data for this Ligand-Target Pair
Affinity DataIC50: 1.44nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.44nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.63nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.63nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.69nMpH: 8.0Assay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.79nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.84nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 1.84nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.99nMpH: 8.0Assay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 2.15nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 2.15nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibition of full length active AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE...More data for this Ligand-Target Pair
Affinity DataIC50: 2.45nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 2.45nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of full length unphosphorylated AKT2 (1 to 481 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen (Amplified Luminesce...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMpH: 8.0 T: 2°CAssay Description:AKT1 activity was assayed using the GSK3-derived biotinylated peptide substrate, crosstide (biotin-GRPRTSSFAEG), and AlphaScreen™ (Amplified Lum...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of full length active AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-GRPRTSSFAE...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of full length unphosphorylated AKT1 (1 to 480 residues) (unknown origin) expressed in baculovirus infected Sf9 insect cells using biotin-...More data for this Ligand-Target Pair