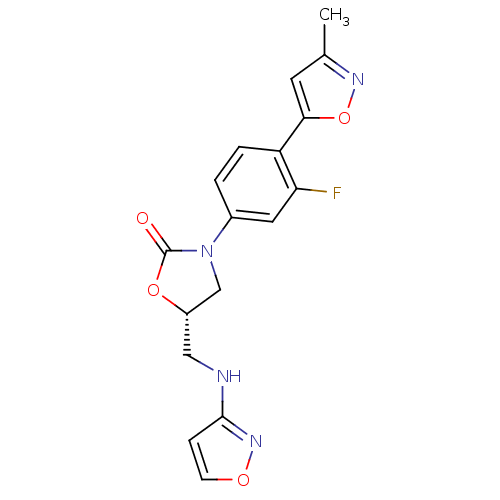
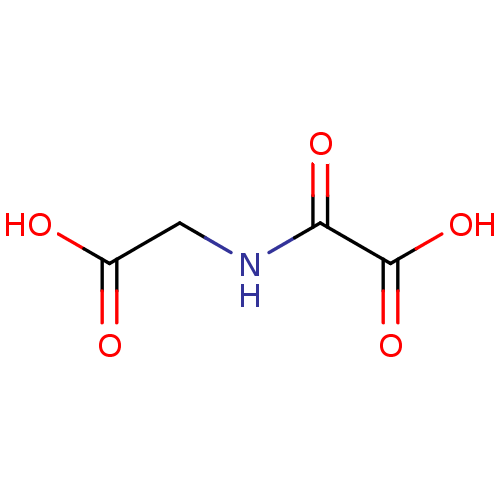
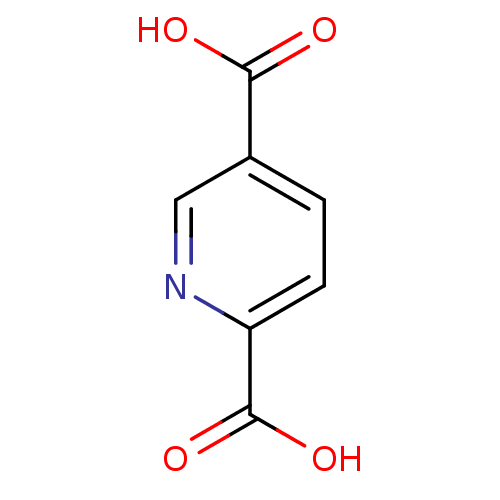
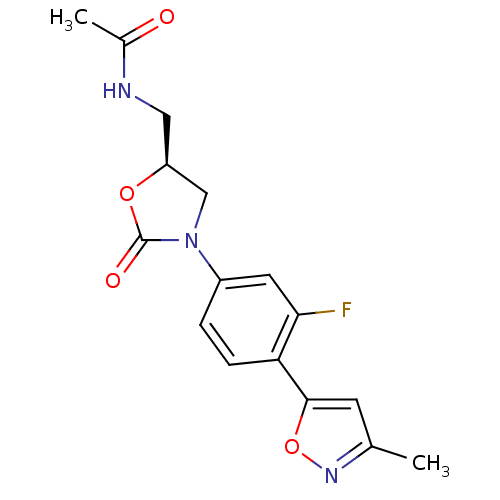
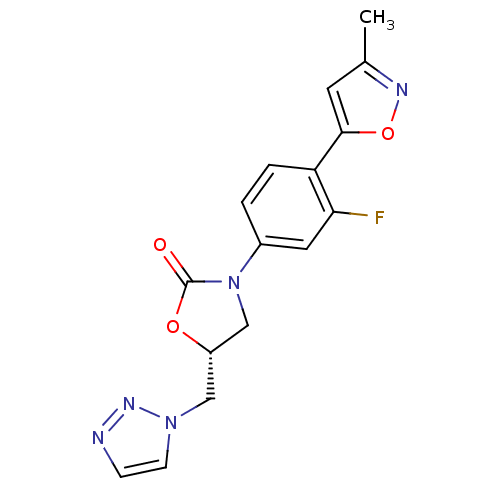
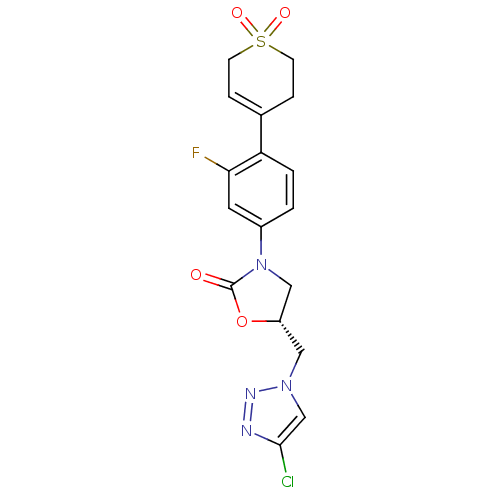
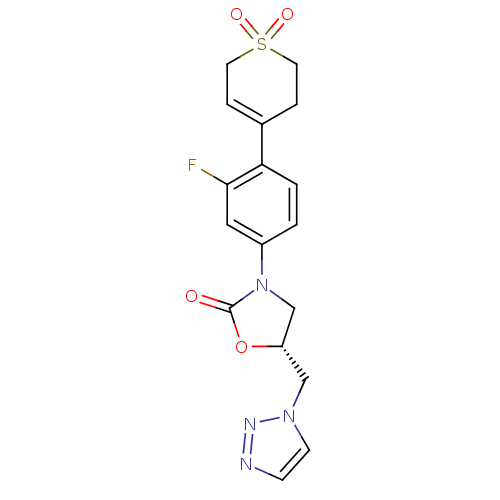
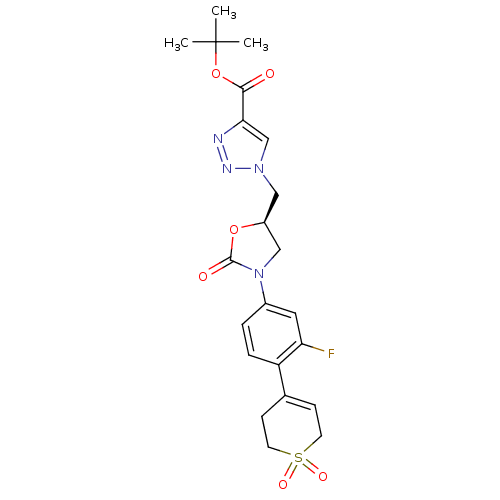
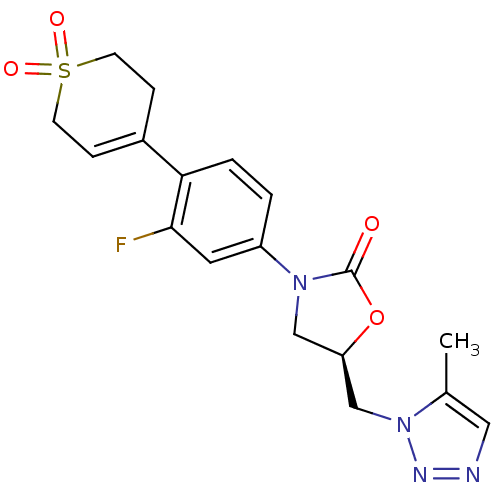
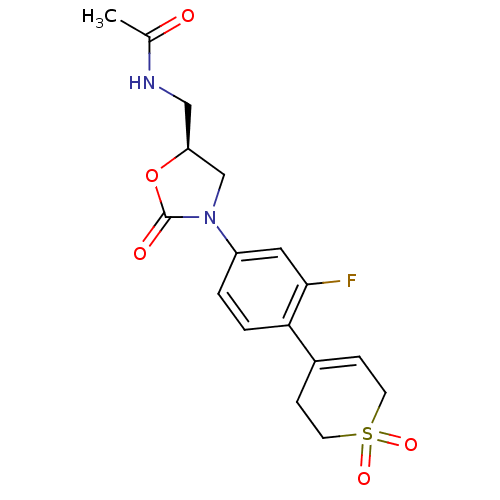
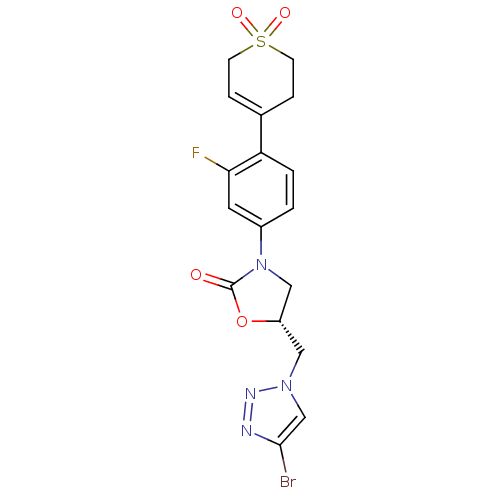
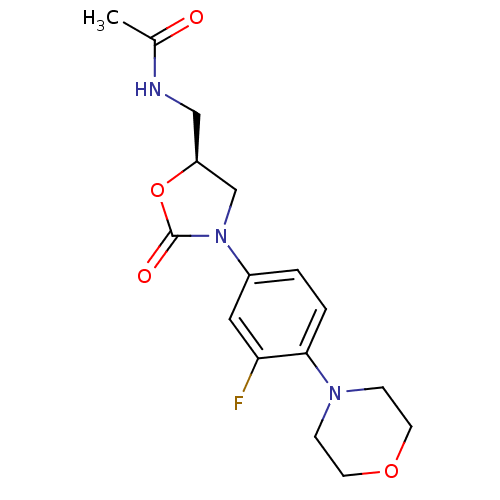
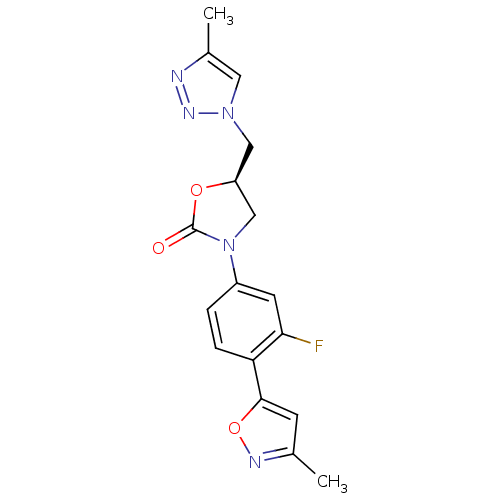
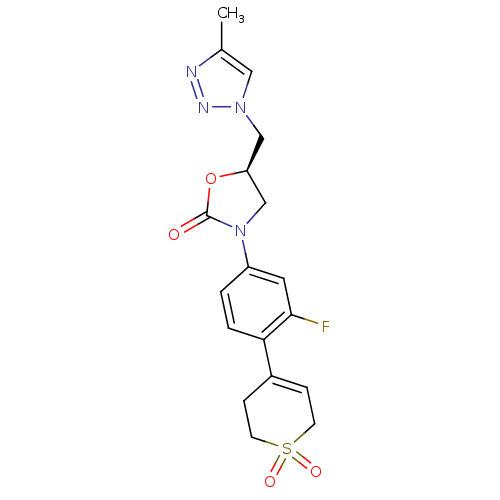
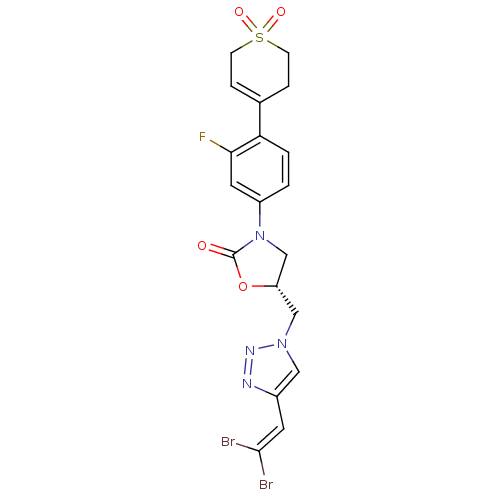
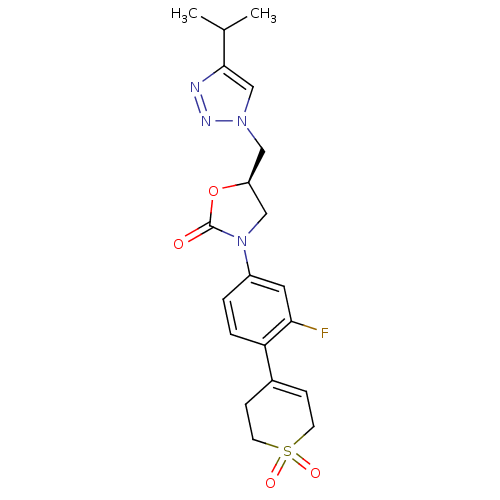
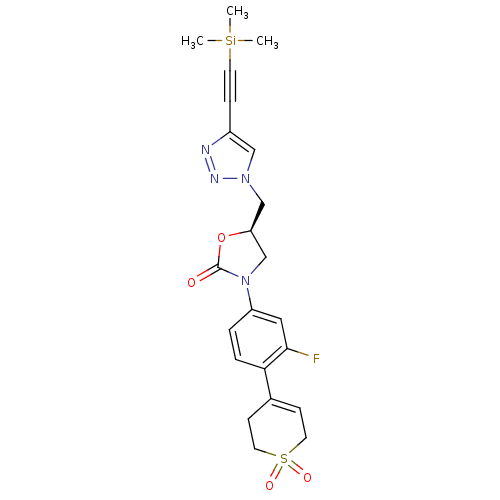
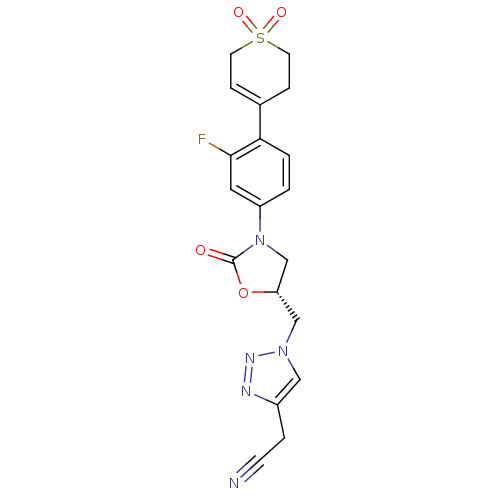
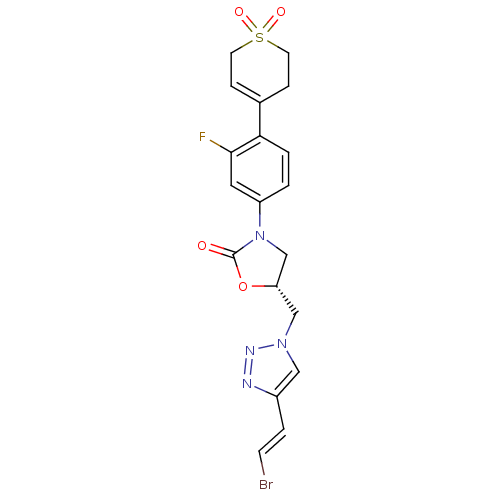
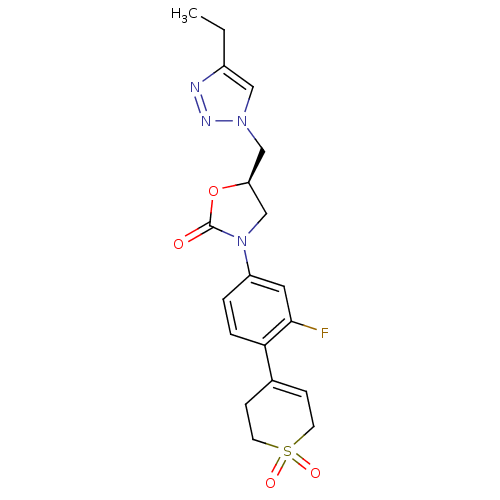
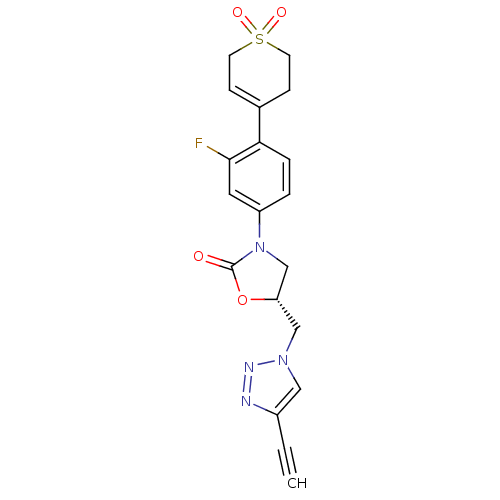
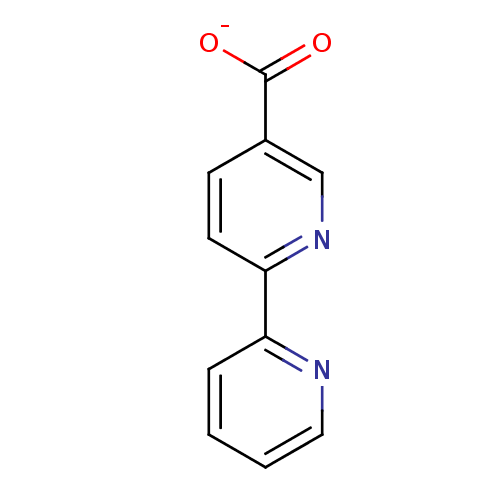
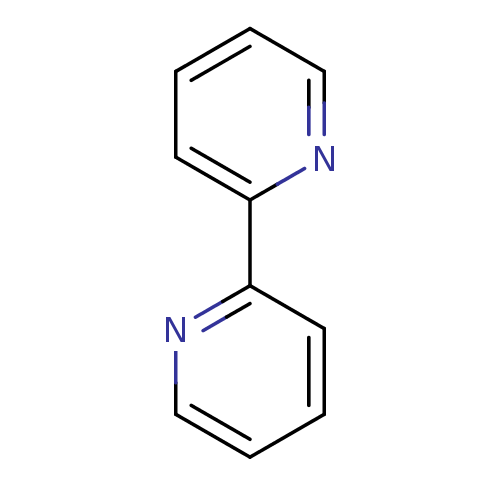
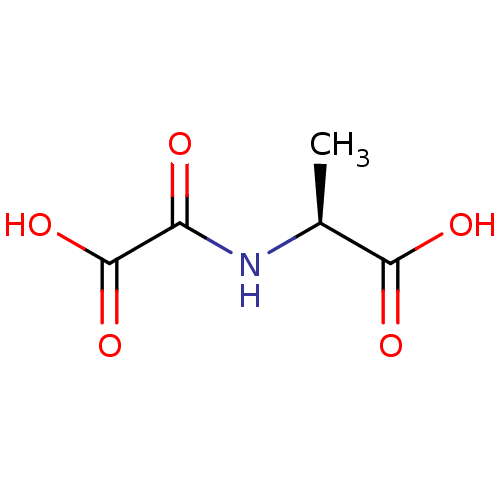
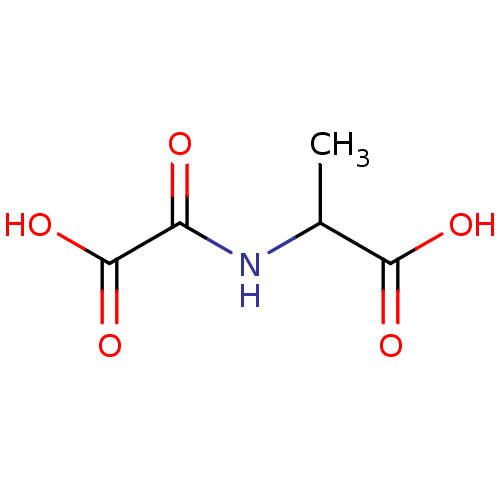
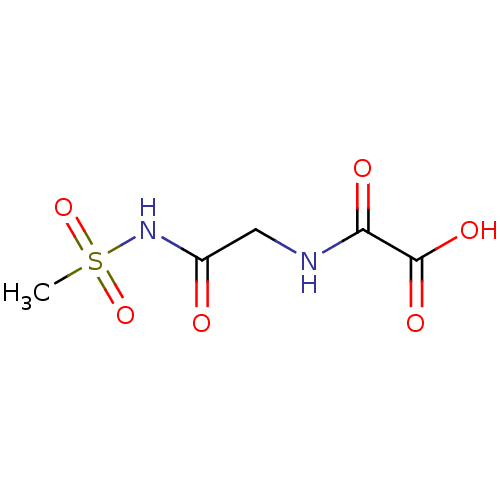
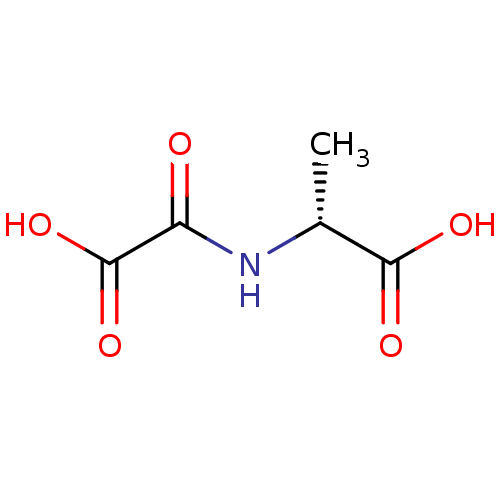
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 420nMAssay Description:Inhibition human liver MAOA activityMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 500nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Inhibitory activity against prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 950nMAssay Description:Inhibition human liver MAOA activityMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 2.90E+3nMAssay Description:Inhibition human liver MAOA activityMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 3.20E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 3.40E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 3.50E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 4.50E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 5.90E+3nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 1.60E+4nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 2.00E+4nMAssay Description:Inhibition human liver MAOA activityMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 2.26E+4nMAssay Description:Inhibition human liver MAOA activityMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 2.50E+4nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 4.70E+4nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: 1.02E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Homo sapiens (Human))
AstraZeneca R&D Boston
Curated by ChEMBL
AstraZeneca R&D Boston
Curated by ChEMBL
Affinity DataKi: >2.00E+5nMAssay Description:Binding affinity for human liver monoamine oxidase AMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 185nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.89E+3nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.04E+3nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 5.18E+3nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
Affinity DataIC50: 5.18E+3nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.32E+4nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.38E+4nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 3.42E+4nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
Affinity DataIC50: 3.82E+4nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 9.07E+4nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
TargetTransmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Zeneca Pharmaceuticals
Curated by ChEMBL
Zeneca Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.12E+5nMAssay Description:Inhibition of prolyl 4-hydroxylase by chromatographic determination of [14C]-succinic acid on ion-exchange minicolumnaMore data for this Ligand-Target Pair
Affinity DataIC50: 4.14E+5nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylaseMore data for this Ligand-Target Pair
Affinity DataIC50: 6.21E+5nMAssay Description:Compound was evaluated for the inhibition of prolyl 4-hydroxylase at 50 ug/mLMore data for this Ligand-Target Pair