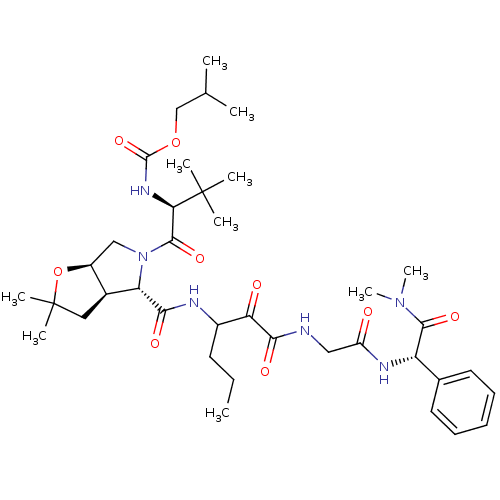
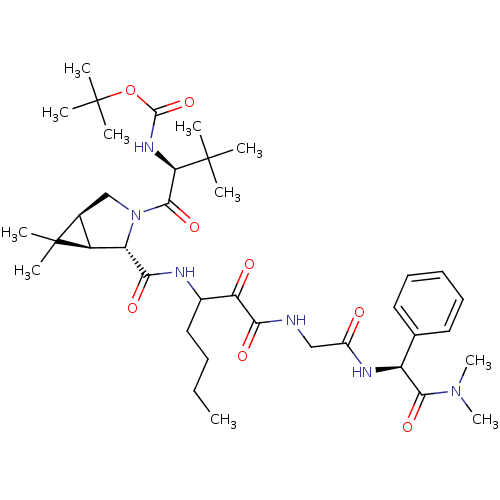
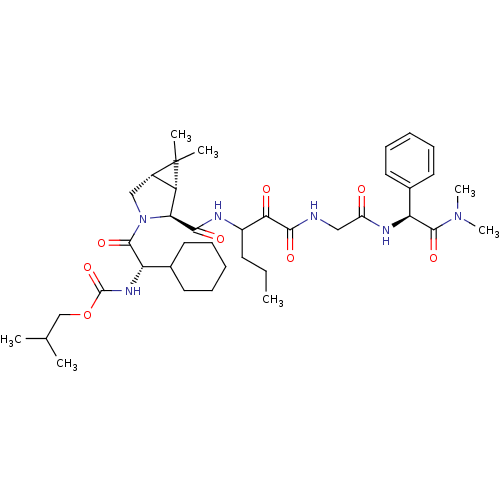
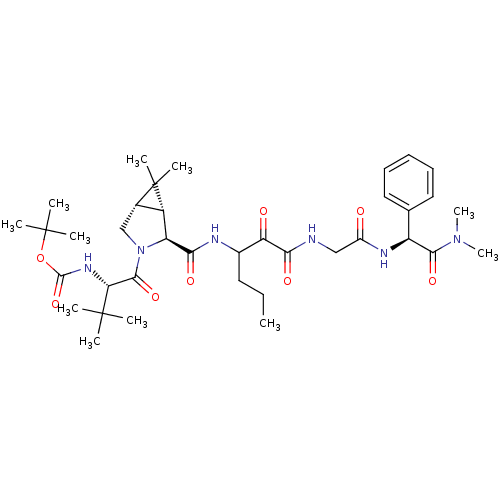
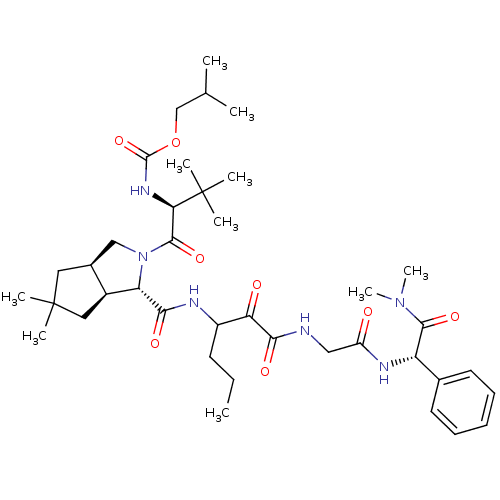
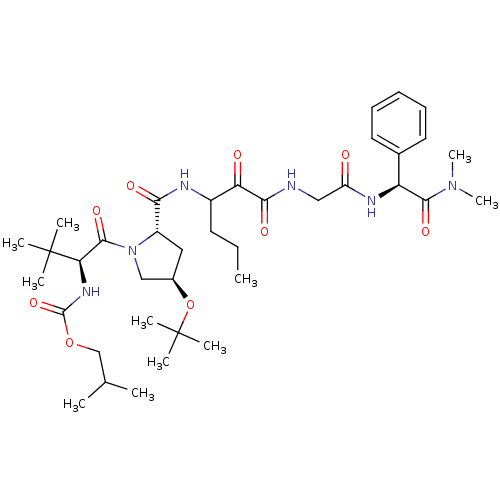
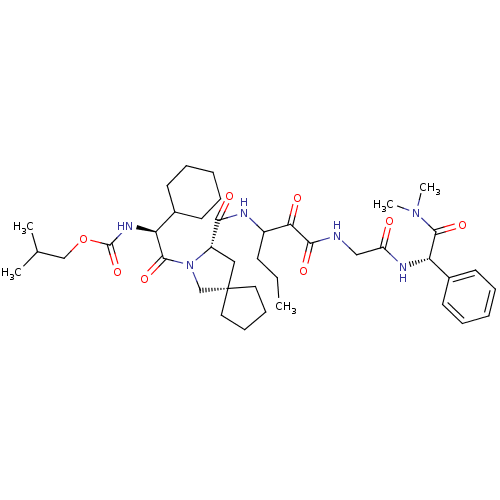
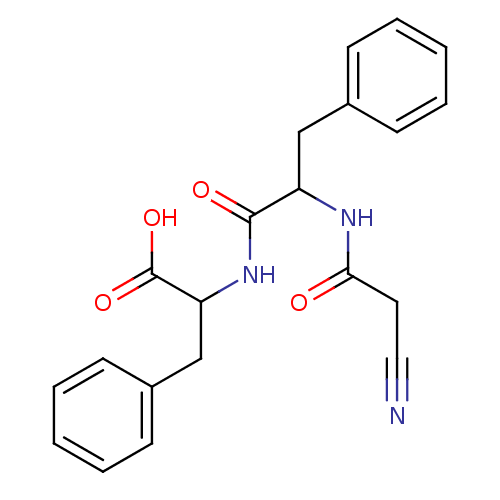
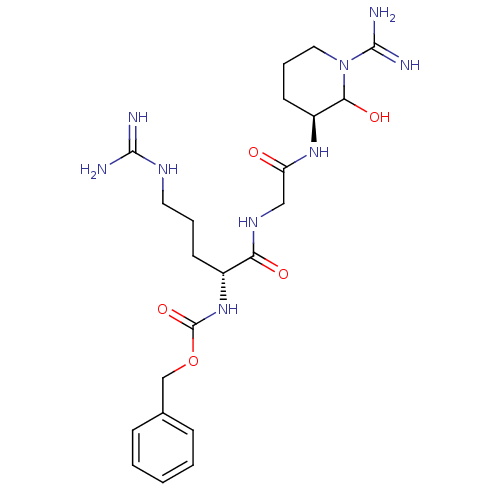
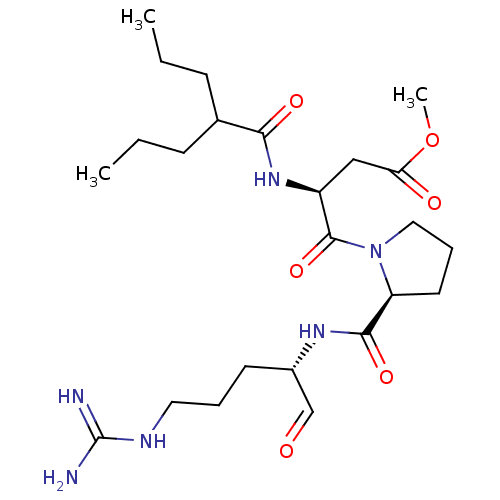
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 2nM ΔG°: -50.5kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
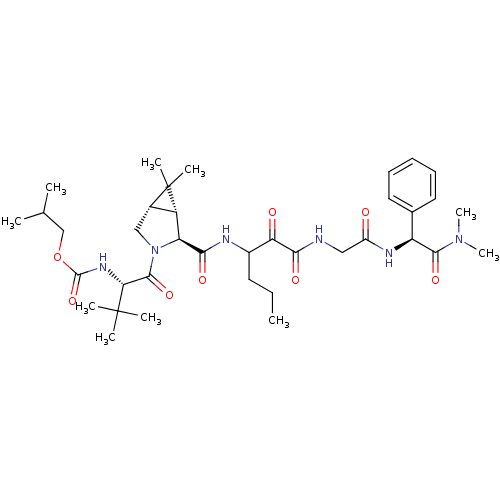
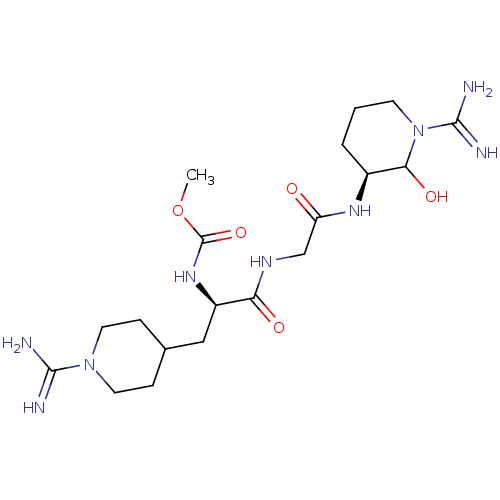
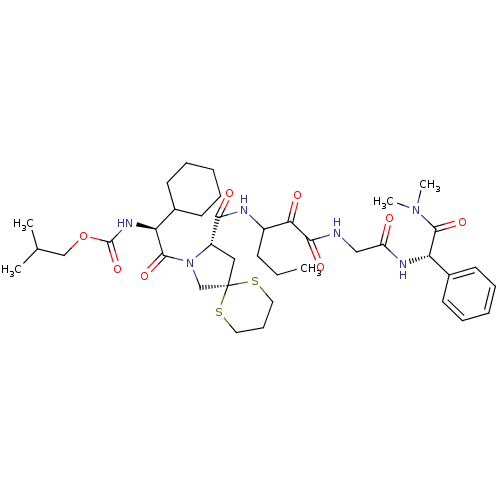
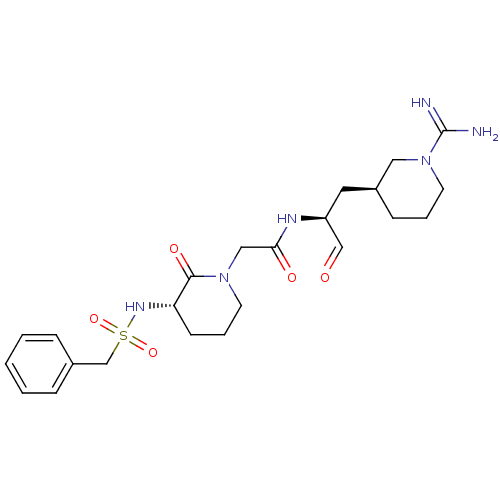
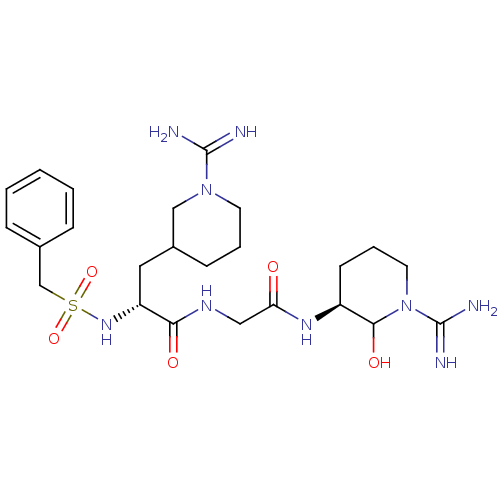
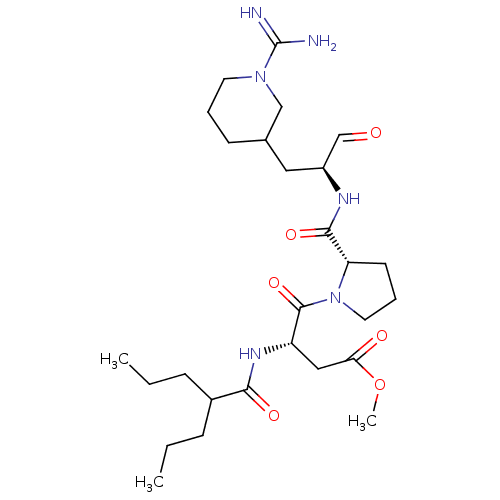
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 3.80nM ΔG°: -48.9kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
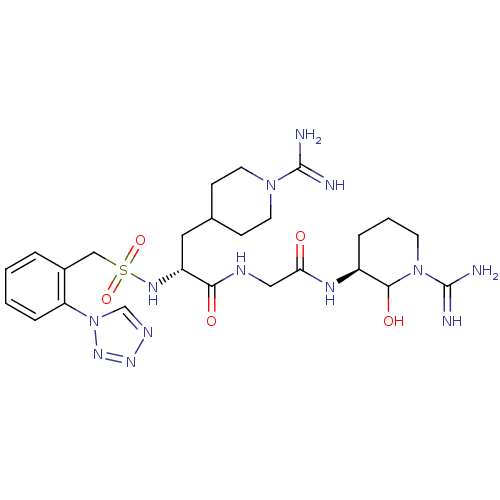
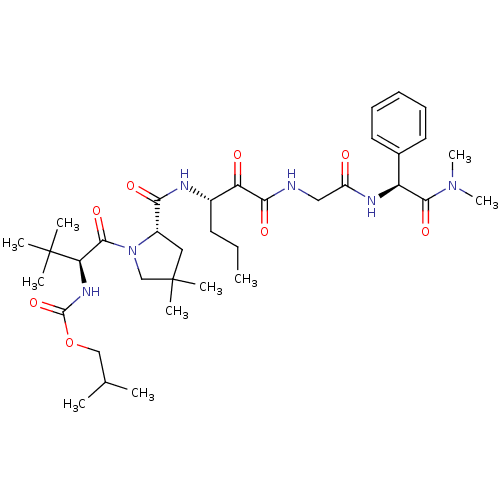
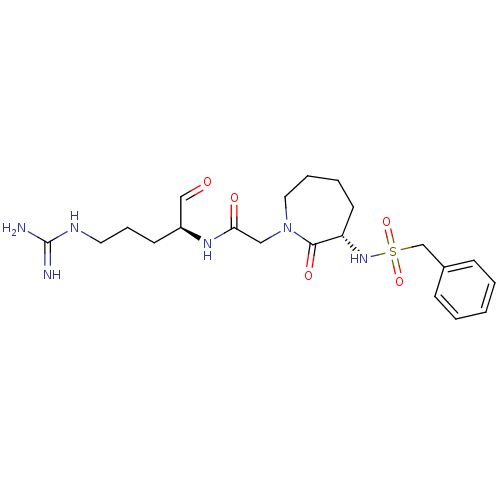
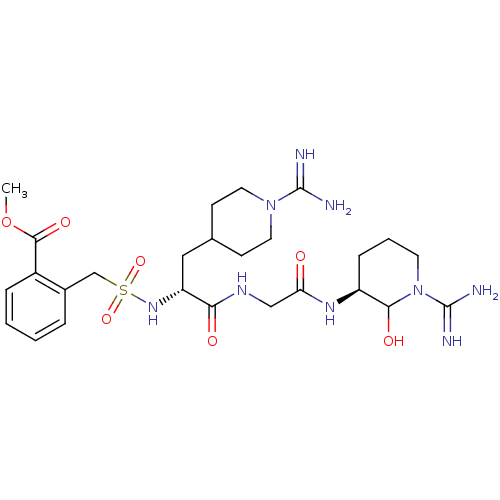
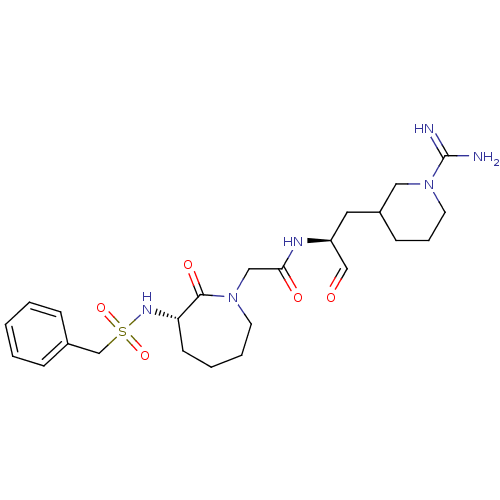
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 10nM ΔG°: -46.4kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
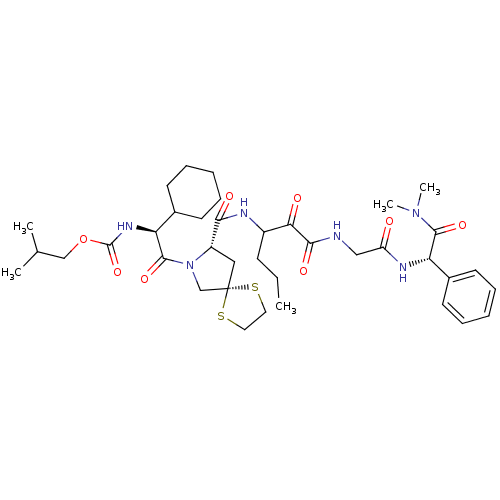
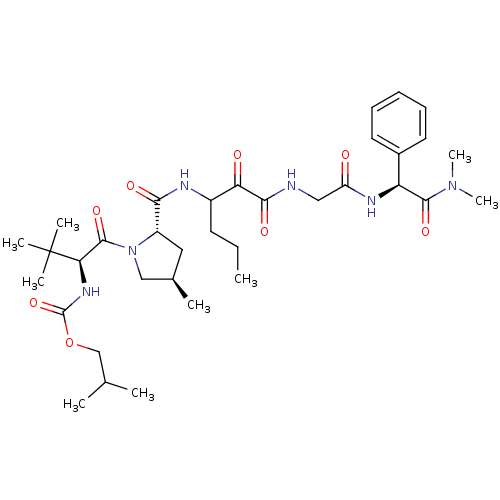
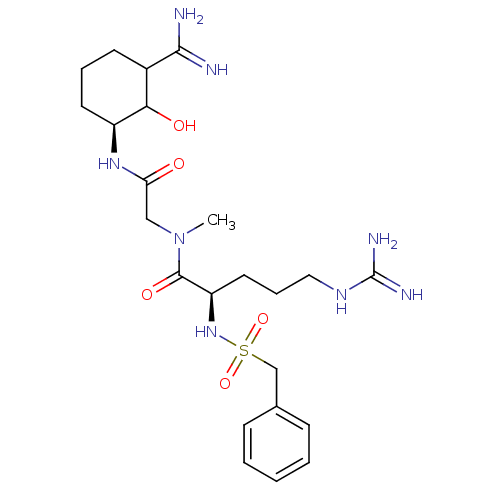
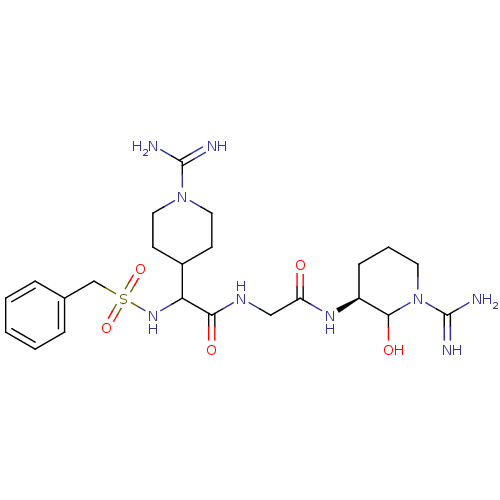
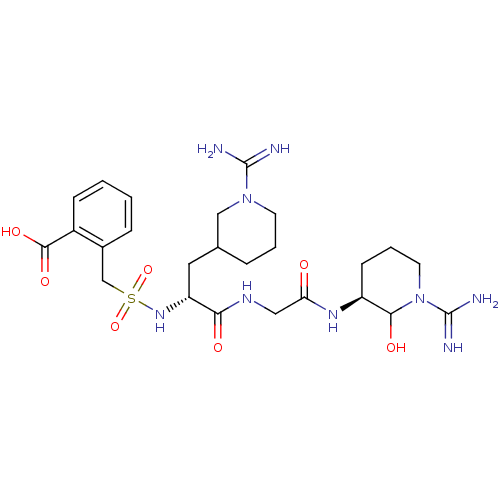
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 10nM ΔG°: -46.4kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 10nM ΔG°: -46.4kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 14nM ΔG°: -45.6kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 15nM ΔG°: -45.4kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
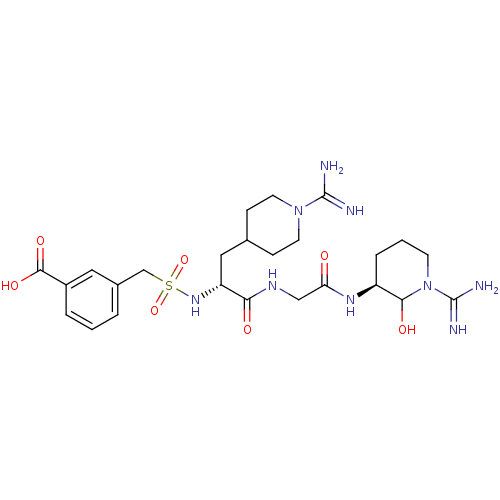
Affinity DataKi: 16nMAssay Description:In vitro inhibition of human trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:In vitro inhibitory activity against human Coagulation factor XMore data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 16nM ΔG°: -45.2kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 19nM ΔG°: -44.8kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
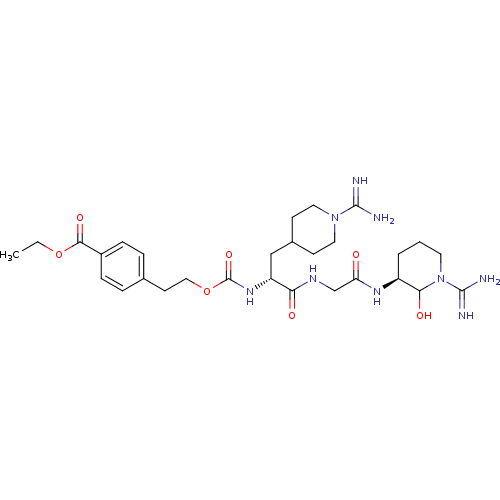
Affinity DataKi: 23nMAssay Description:In vitro inhibitory activity against human Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Concentration of the compound required for classical fast inhibition of cleavage of the chromogenic substrate by human enzyme Coagulation factor X in...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 26nM ΔG°: -44.0kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 26nM ΔG°: -44.0kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 28nM ΔG°: -43.8kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 36nM ΔG°: -43.2kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 120nM ΔG°: -40.2kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 140nM ΔG°: -39.8kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
TargetGenome polyprotein(Hepatitis C virus (HCV genotype 1a, isolate H))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataKi: 1.00E+4nM ΔG°: -29.0kJ/molepH: 6.5 T: 2°CAssay Description:Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Inhibition constant determined for neutral endopeptidase (NEP)More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Inhibition constant determined for Neutral endopeptidase (NEP)More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Rattus norvegicus)
Salk Biotechnology/Industrial Associates
Curated by ChEMBL
Salk Biotechnology/Industrial Associates
Curated by ChEMBL
Affinity DataKi: 6.50E+4nMAssay Description:Inhibition constant determined for Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Rattus norvegicus)
Salk Biotechnology/Industrial Associates
Curated by ChEMBL
Salk Biotechnology/Industrial Associates
Curated by ChEMBL
Affinity DataKi: >1.00E+7nMAssay Description:Inhibition constant determined for angiotensin converting enzyme (ACE)More data for this Ligand-Target Pair
Affinity DataIC50: 0.670nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:In vitro inhibition of human Factor Xa.More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 0.830nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.860nMAssay Description:Inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Inhibition of human VCAM and Ramos cell VLA-4 interactionMore data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate trypsinMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 1.37nMAssay Description:Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Compound was evaluated for inhibition of amidolytic activity for chromogenic substrate thrombin F11a.More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibitory activity against Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human VCAM binding to VLA-4 of Ramos cells in ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:In vitro inhibition of human Coagulation factor X.More data for this Ligand-Target Pair

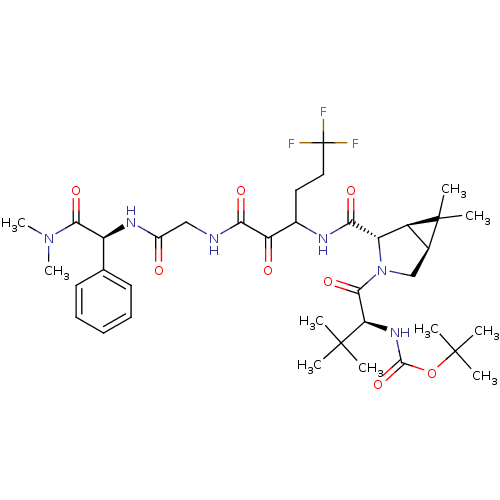
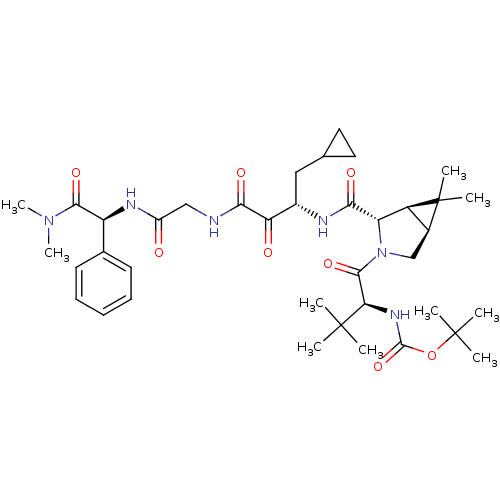
 3D Structure (crystal)
3D Structure (crystal)