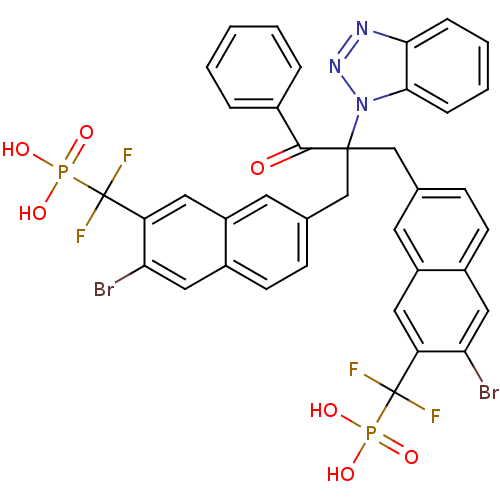
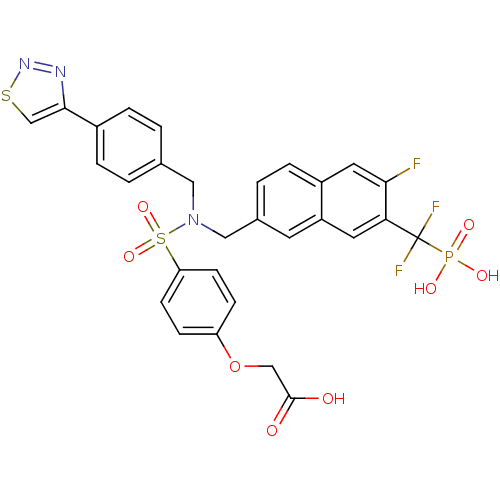
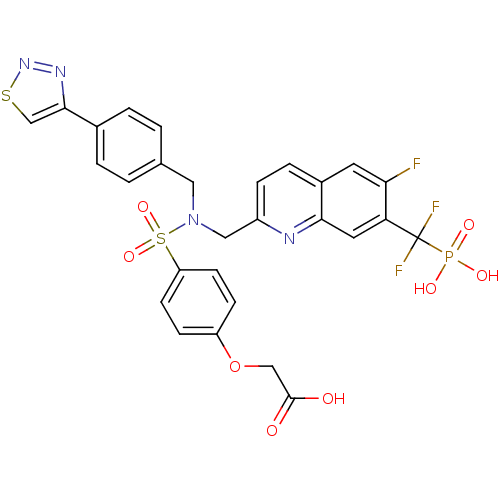
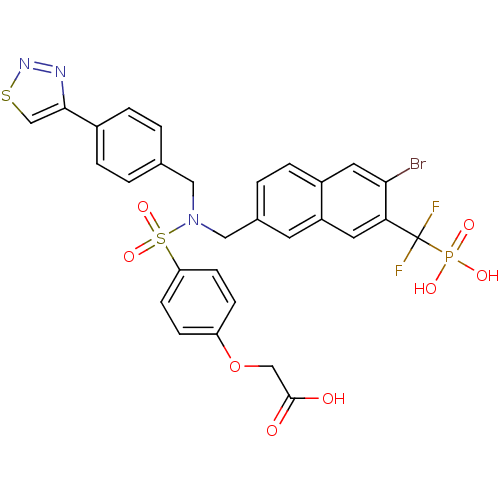
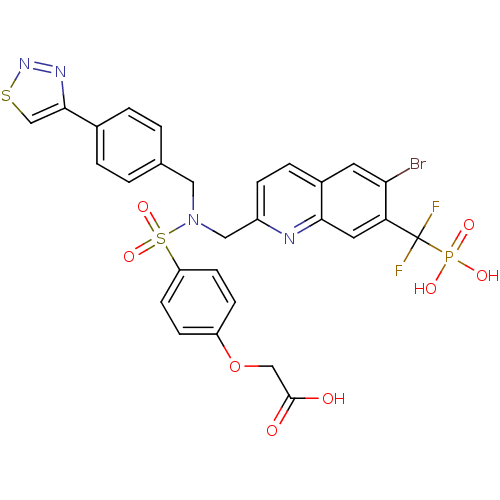
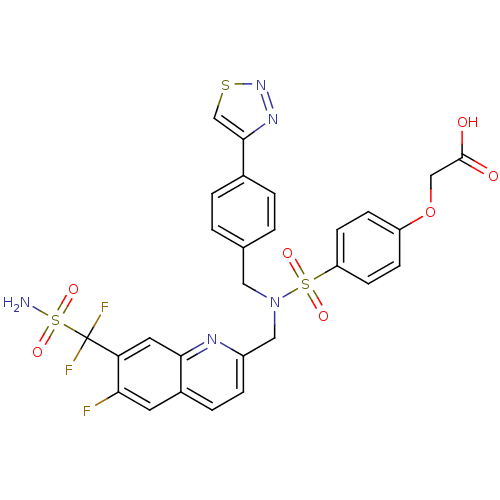
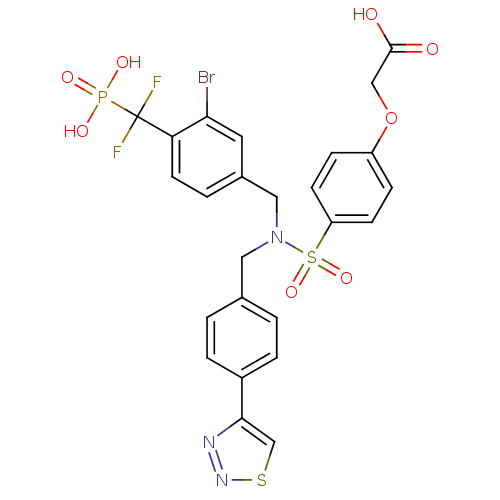
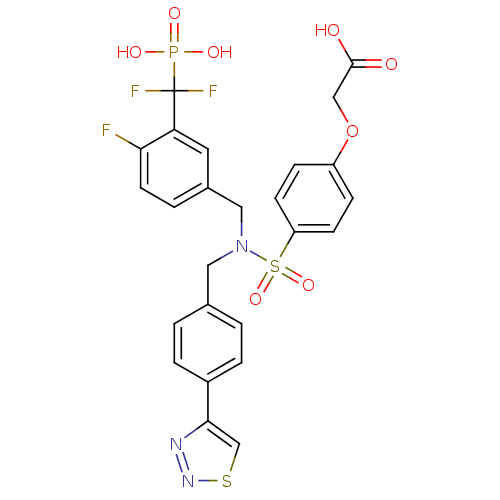
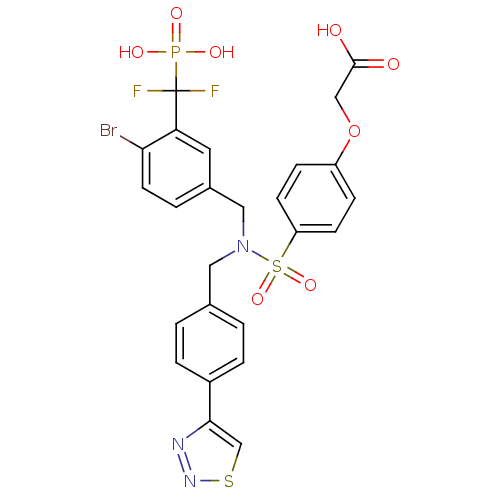
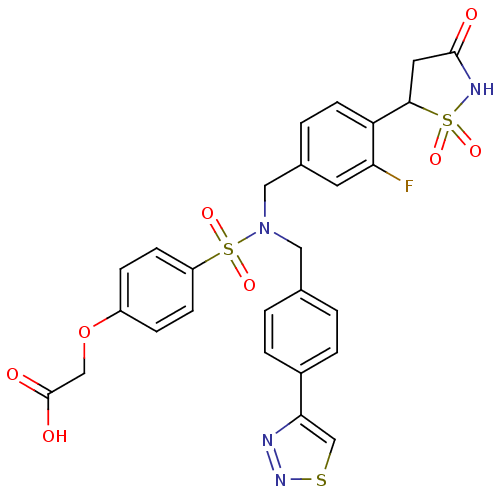
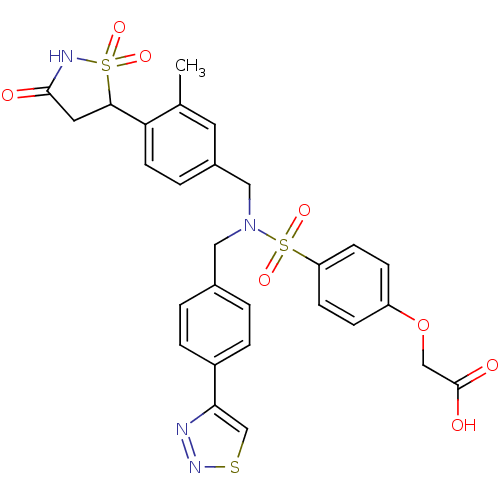
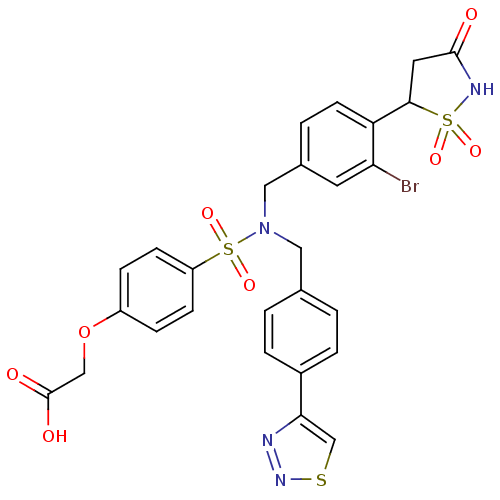
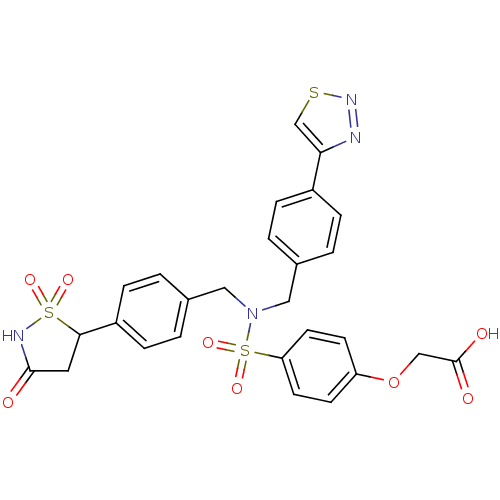
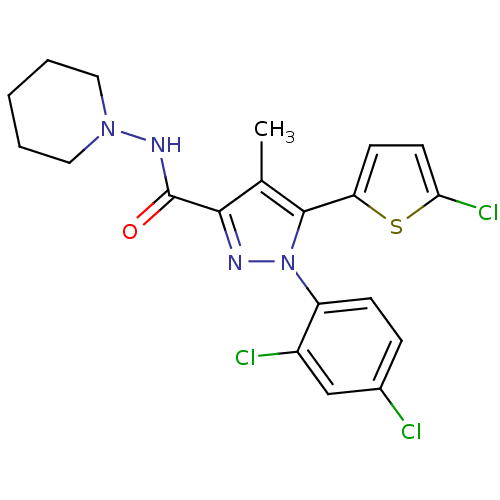
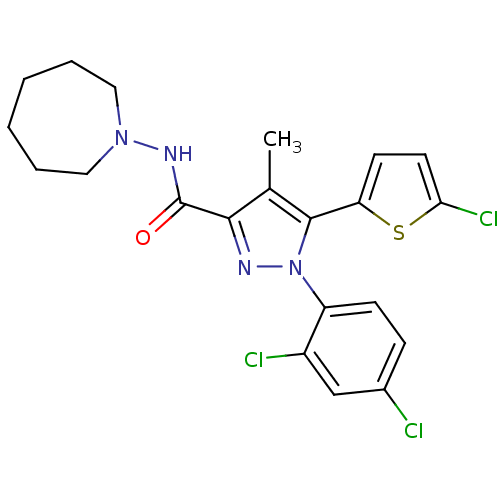
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
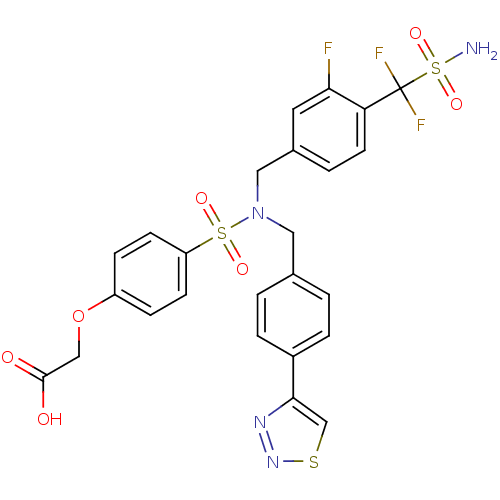
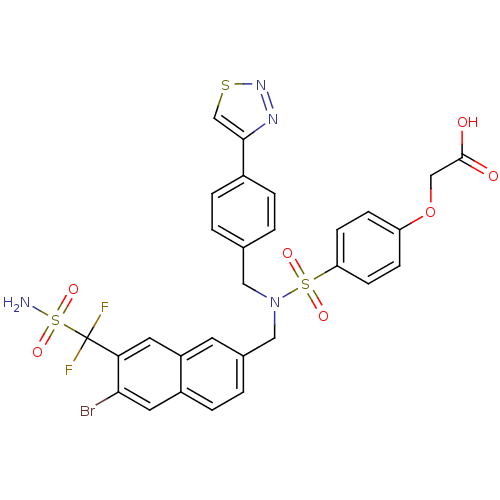
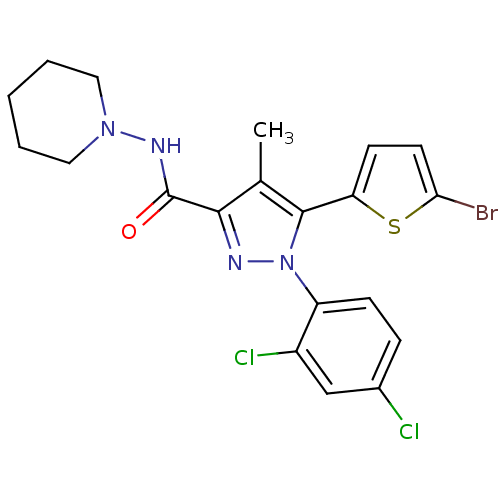
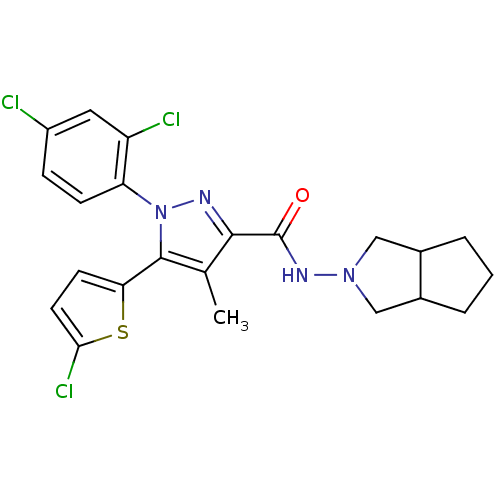
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
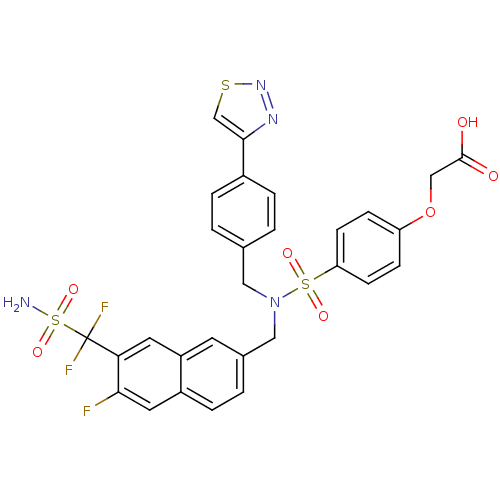
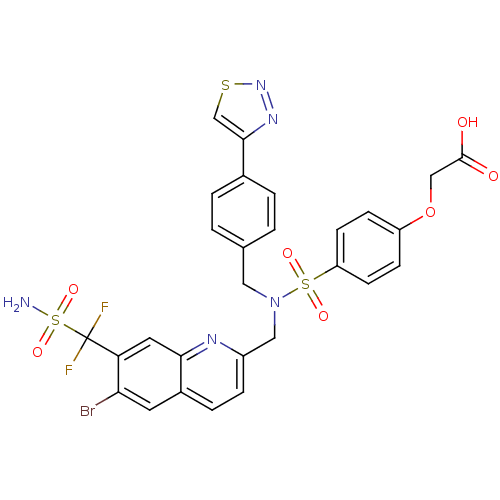
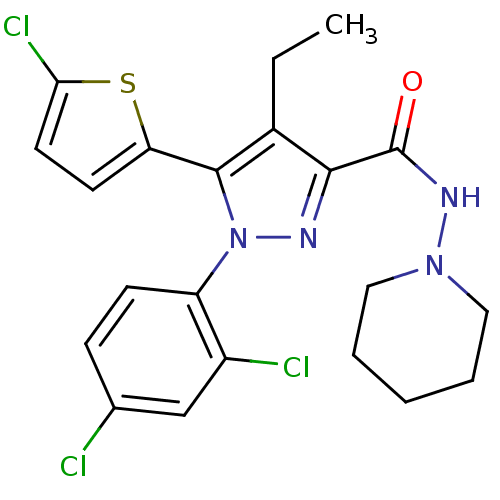
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
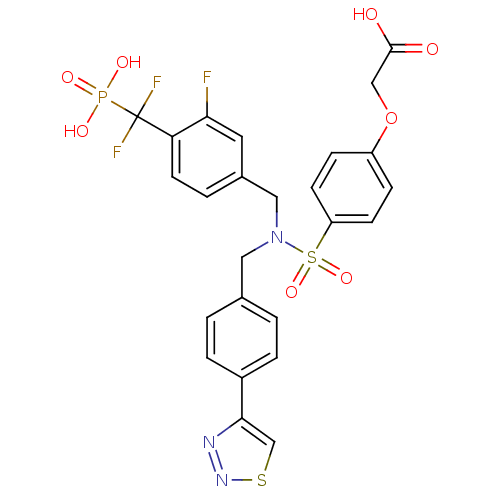
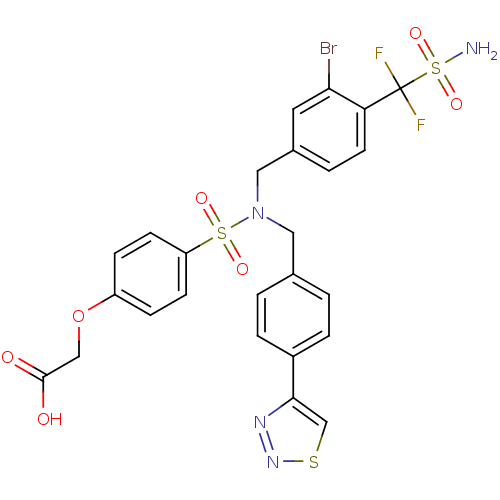
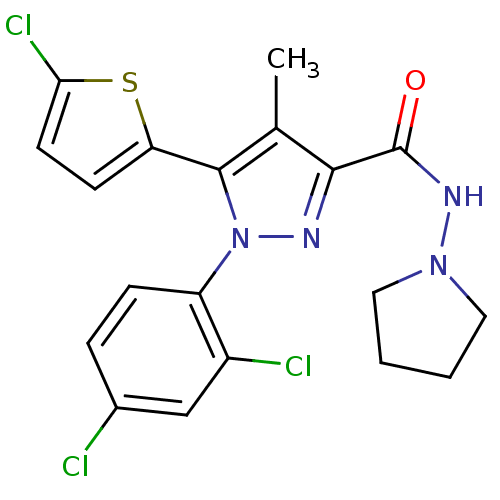
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 38nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 39nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 47nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 141nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 288nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 470nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of PTP1B assessed as para-nitrophenyl phosphate catalyzed hydrolysis of para-nitrophenol by p-NPP assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
TargetTyrosine-protein phosphatase non-receptor type 2(Homo sapiens (Human))
M.S. University of Baroda
Curated by ChEMBL
M.S. University of Baroda
Curated by ChEMBL
Affinity DataEC50: 7.43E+3nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 7.55E+3nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 580nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 2.37E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 400nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 2.32E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 320nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 2.94E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 540nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 2.59E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 1.95E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 510nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 240nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 3.12E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+3nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 2.09E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10E+3nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 1.28E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 690nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 800nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 1.57E+4nMAssay Description:Antagonist activity at human cannabinoid CB2 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair
Affinity DataEC50: 1.33E+3nMAssay Description:Antagonist activity at human cannabinoid CB1 receptor expressed in CHOK1 cells assessed as cAMP activityMore data for this Ligand-Target Pair