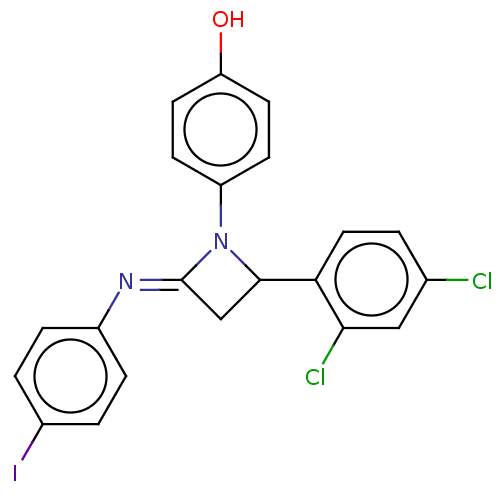
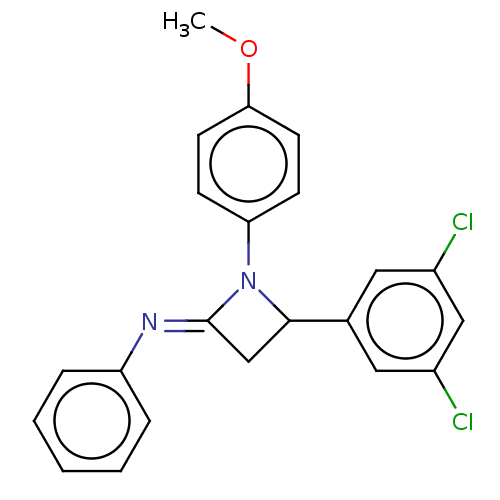
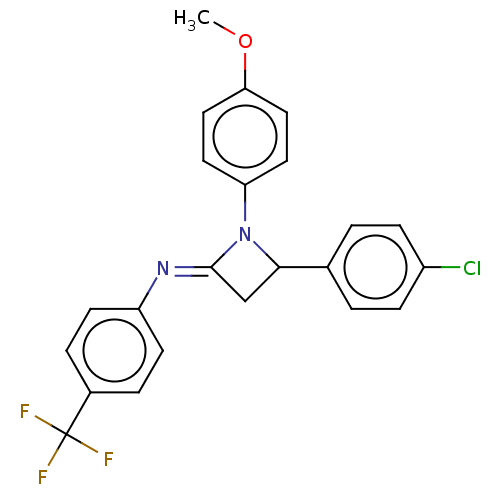
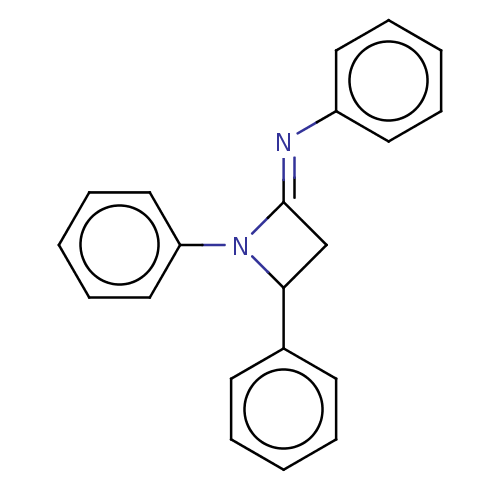
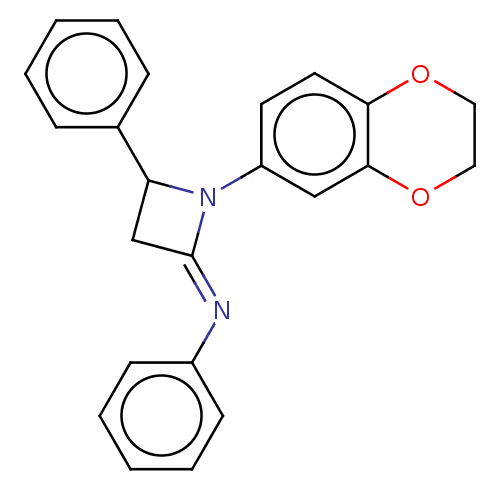
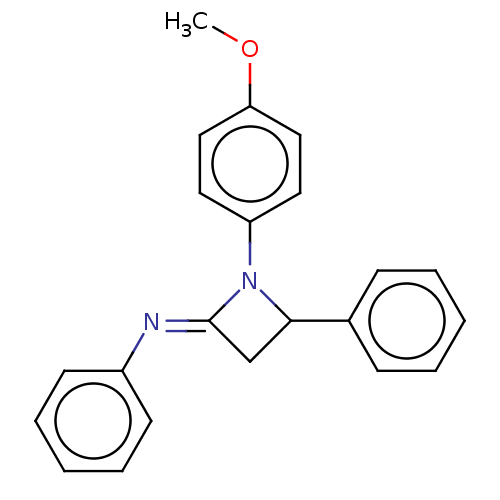
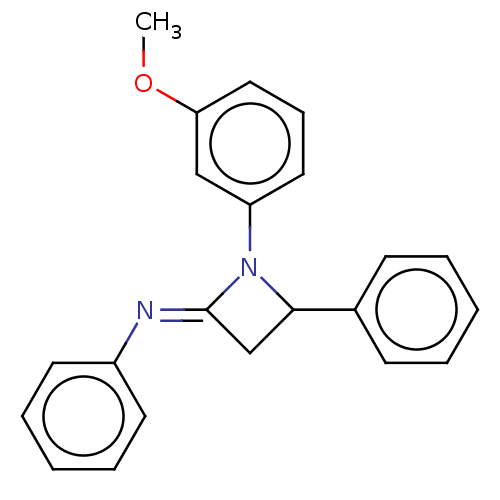
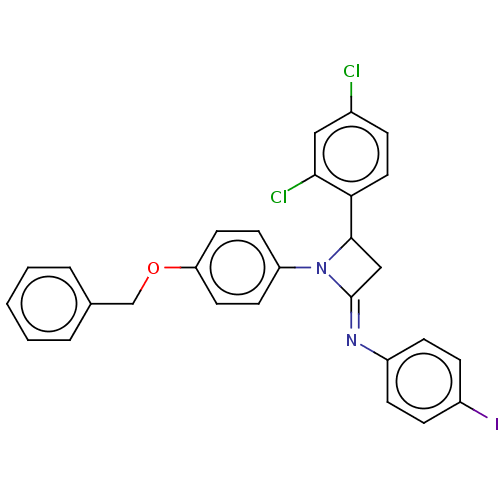
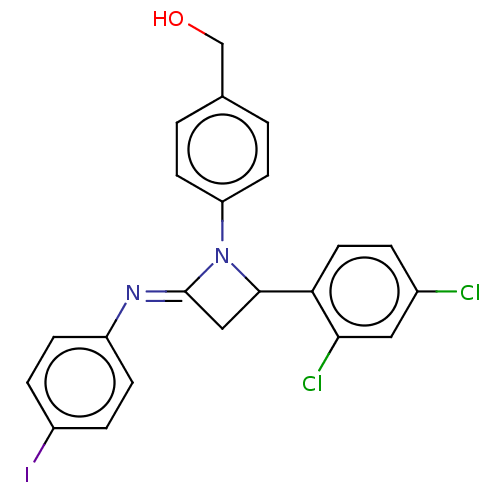
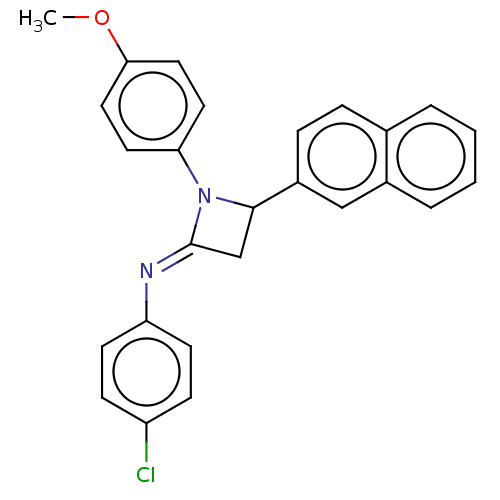
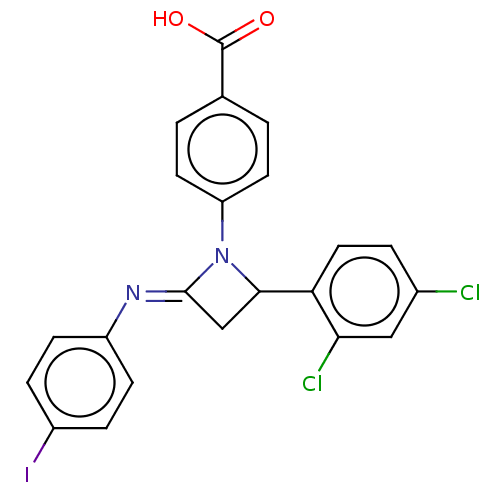
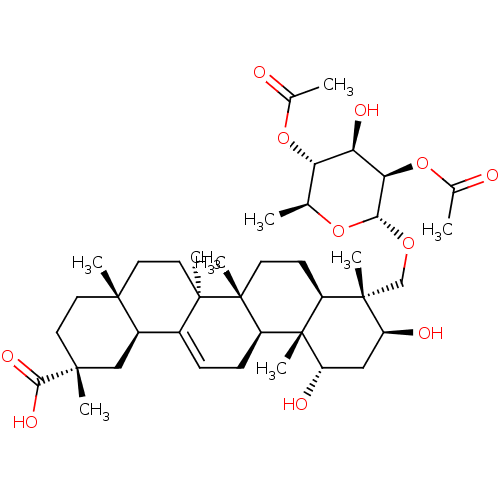
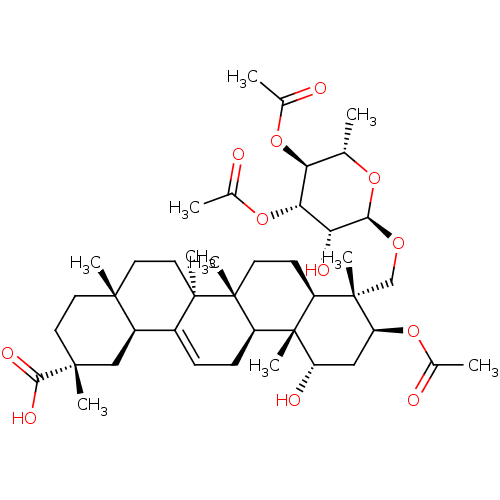
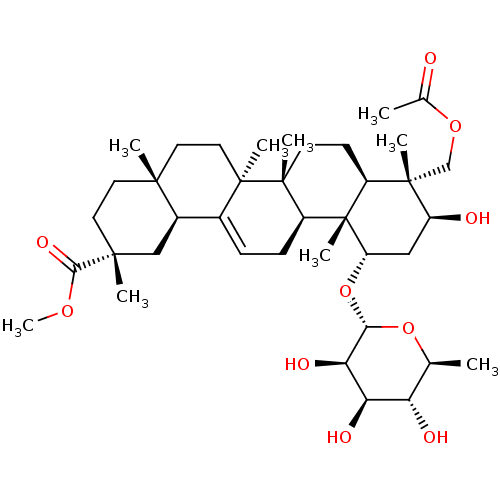
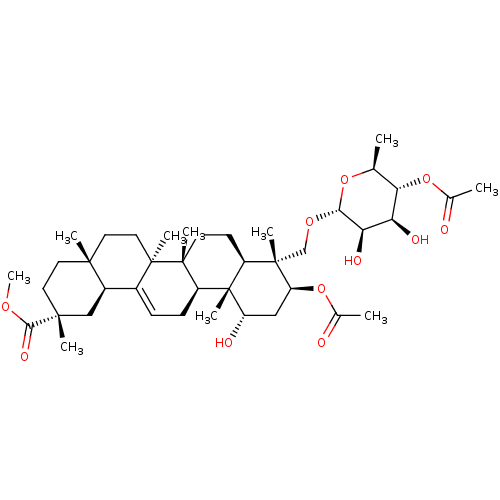
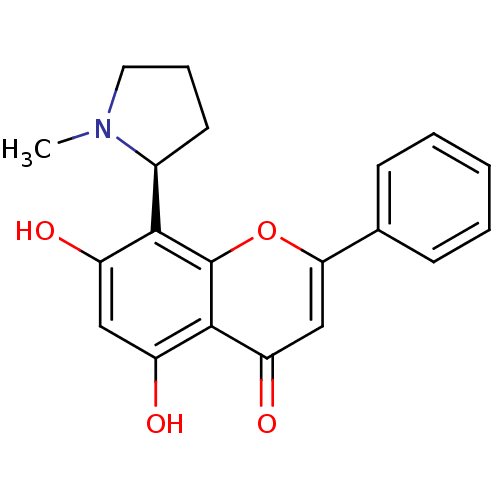
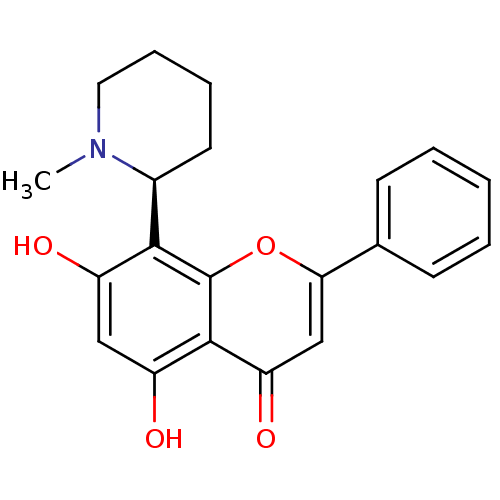
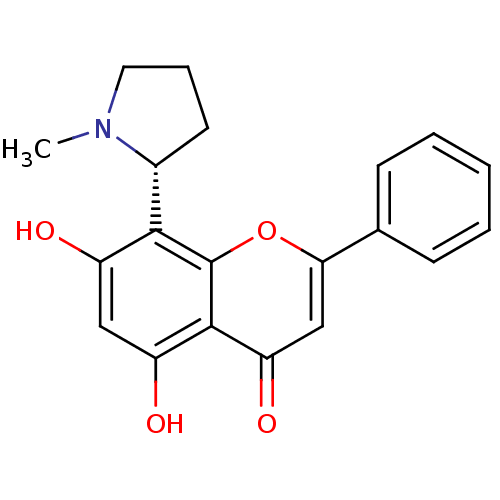
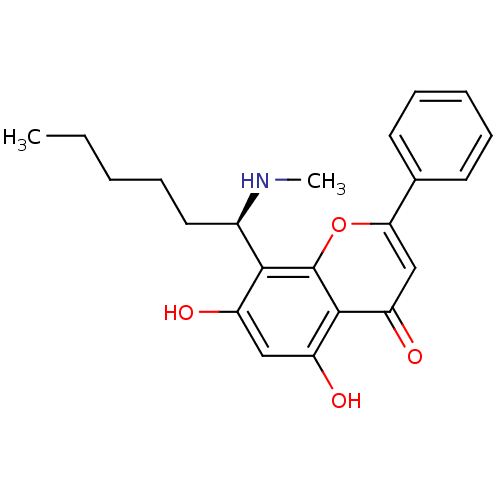
Affinity DataKi: 280nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
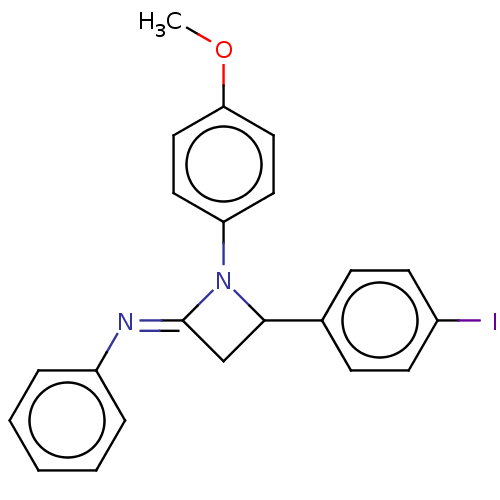
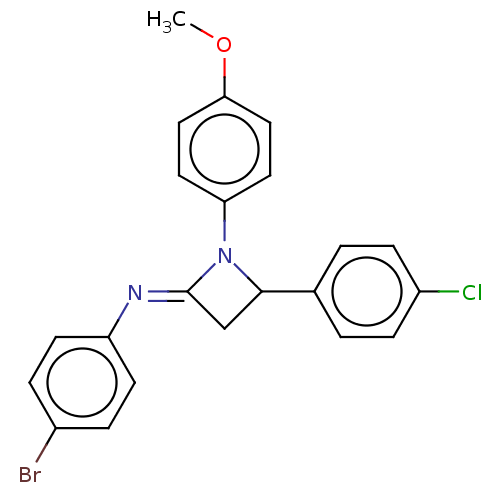
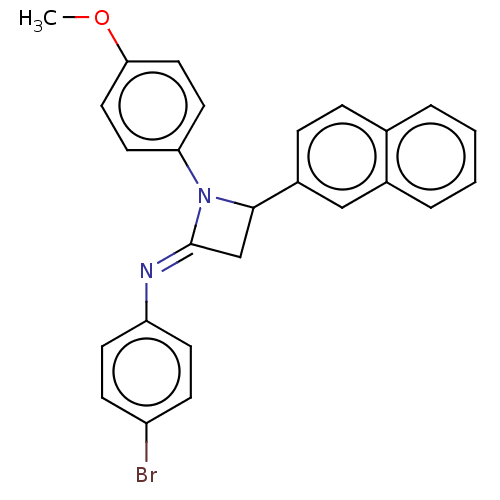
Affinity DataKi: 730nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
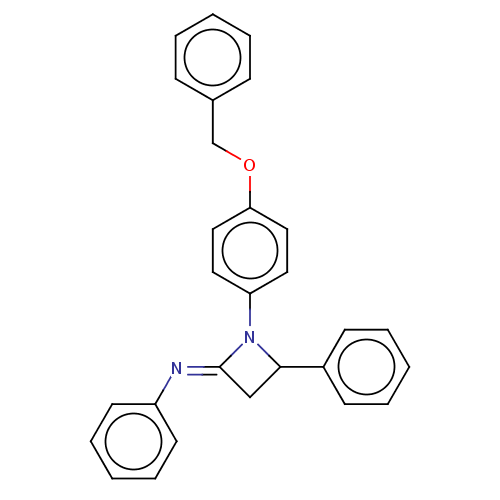
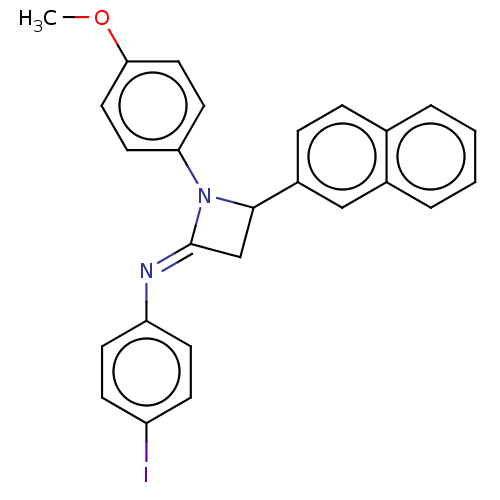
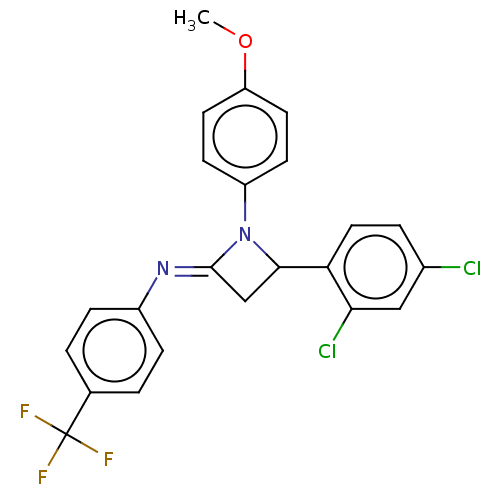
Affinity DataKi: 1.15E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
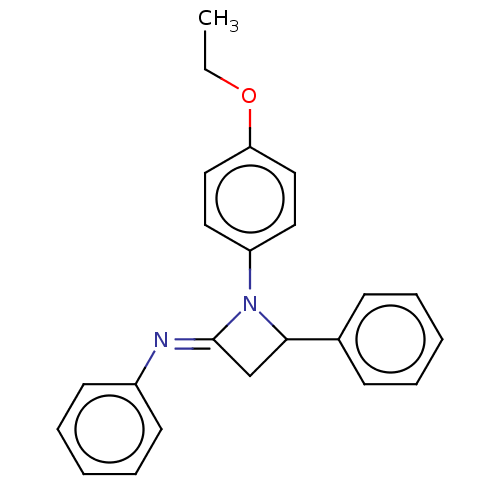
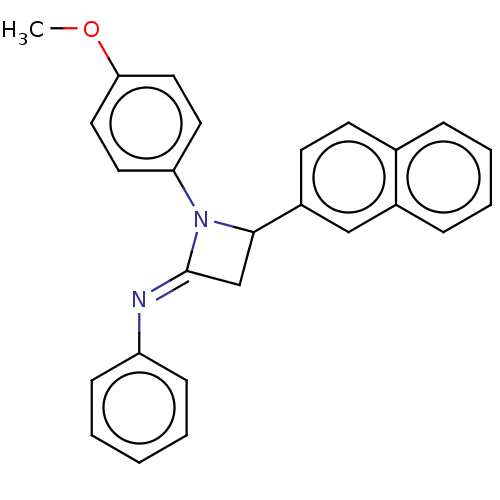
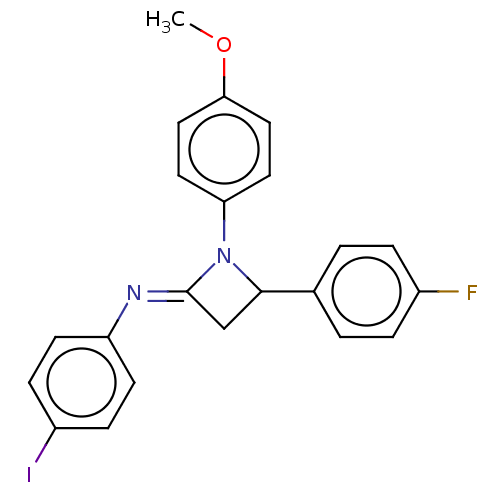
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.55E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.58E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.77E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.81E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.84E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.89E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.93E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.96E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.20E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.36E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.41E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.69E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.89E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.94E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.15E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.23E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 5.66E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.04E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 6.49E+3nMAssay Description:Inhibition of KPC-2 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.20E+3nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 7.60E+3nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 1.41E+4nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: 1.78E+4nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity to Bcl-xL by fluorescein-labeled Bak-BH3 peptide competition binding assayChecked by AuthorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(RAT)
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of GST-tagged rat recombinant DYRK1A expressed in Escherichia coli using KKISGRLSPIMTEQ as substrateMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 520nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 5 activator 1(Homo sapiens (Human))
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 570nMAssay Description:Inhibition of human recombinant CDK5/p25 using [gamma 33P]ATP after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetDual specificity protein kinase CLK1(Mus musculus)
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Institut de Chimie des Substances Naturelles (ICSN)
Curated by ChEMBL
Affinity DataIC50: 670nMAssay Description:Inhibition of GST-tagged mouse recombinant CLK1 expressed in Escherichia coli using GRSRSRSRSRSR as substrateMore data for this Ligand-Target Pair