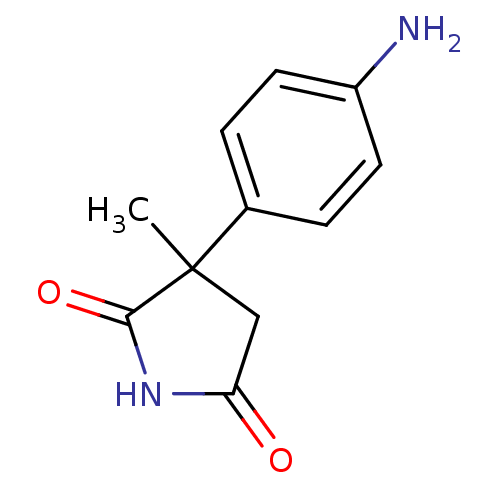
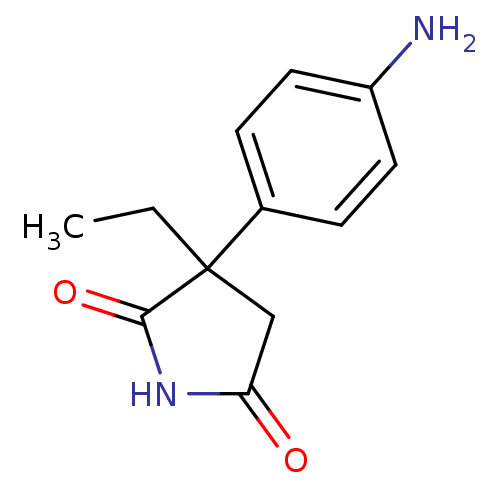
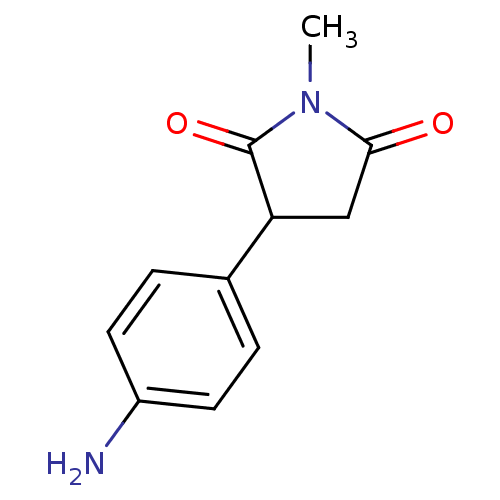
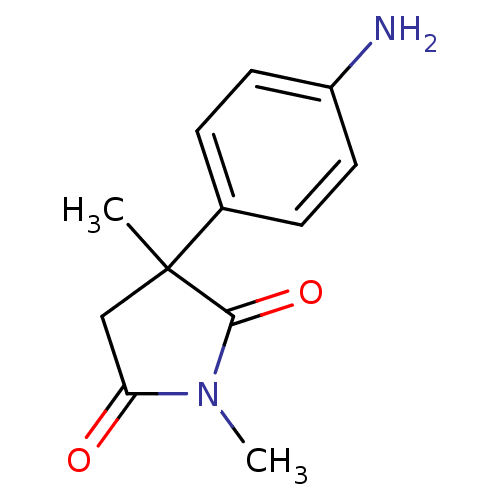
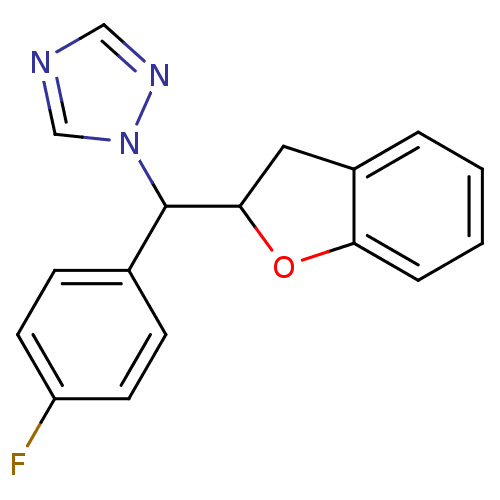
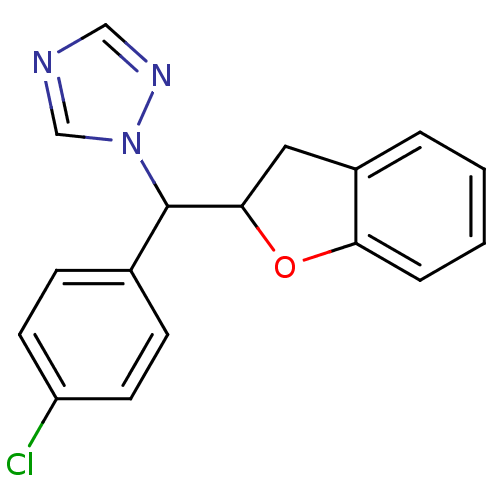
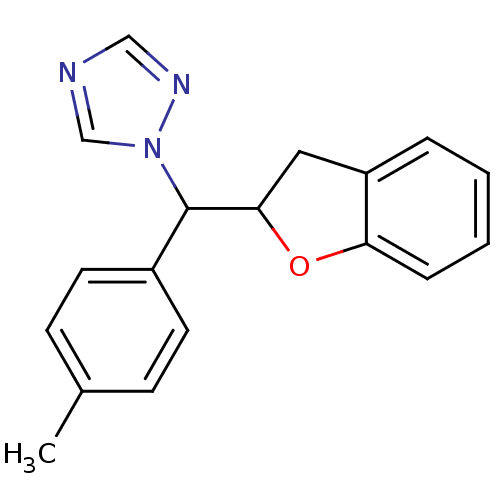
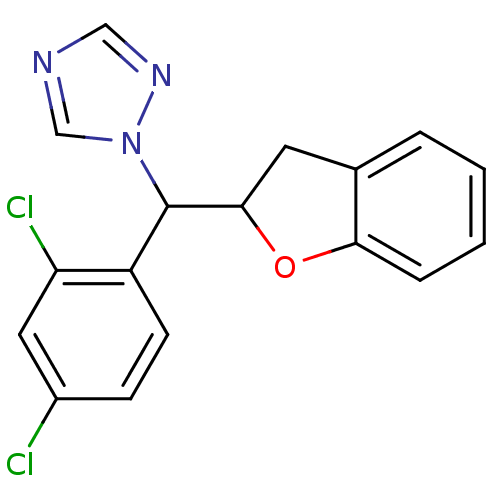
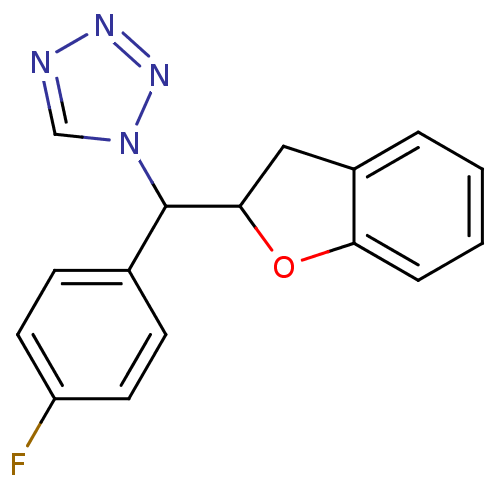
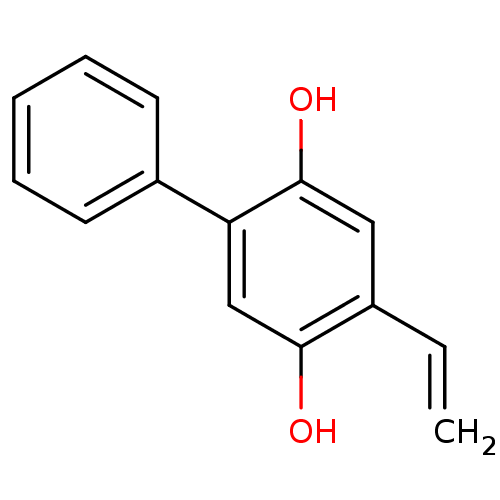
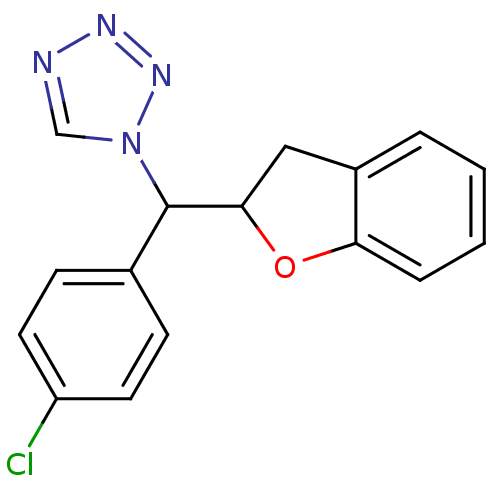
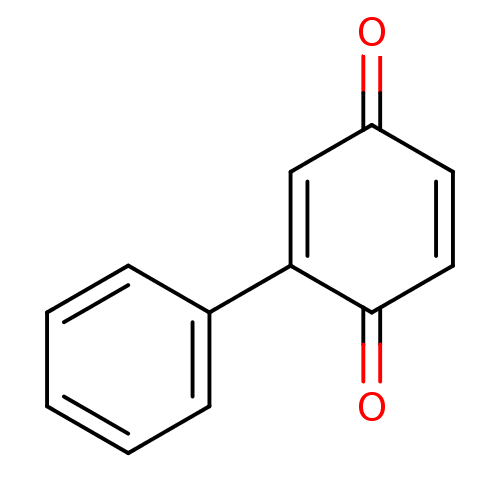
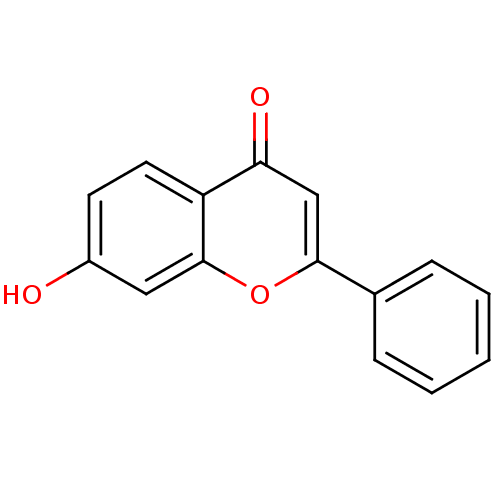
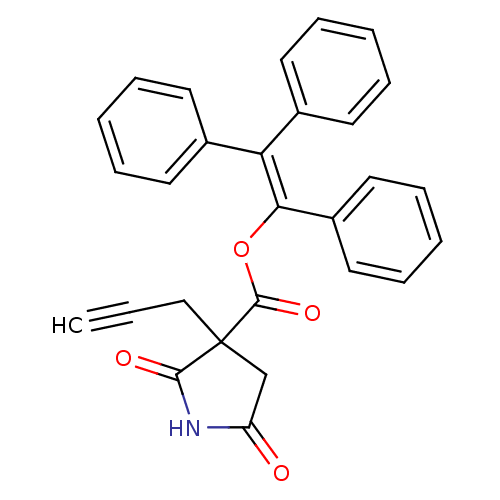
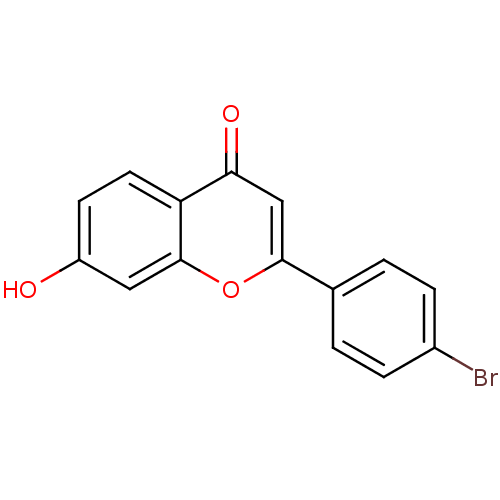
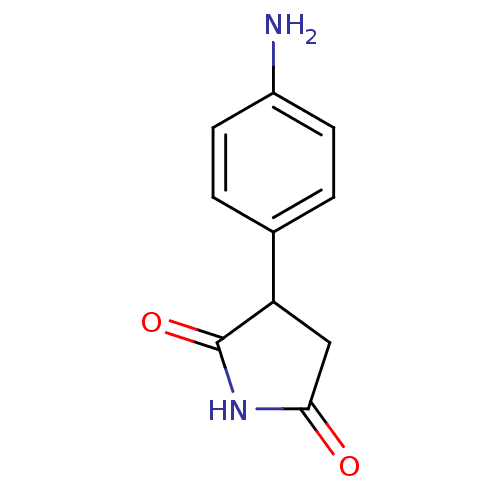
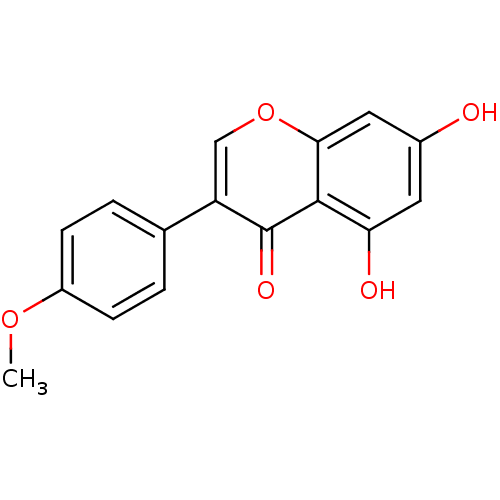
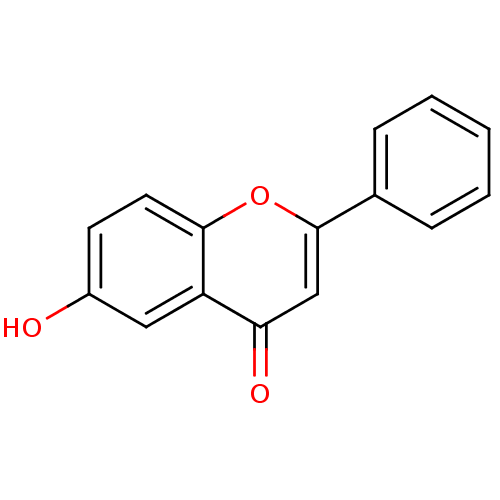
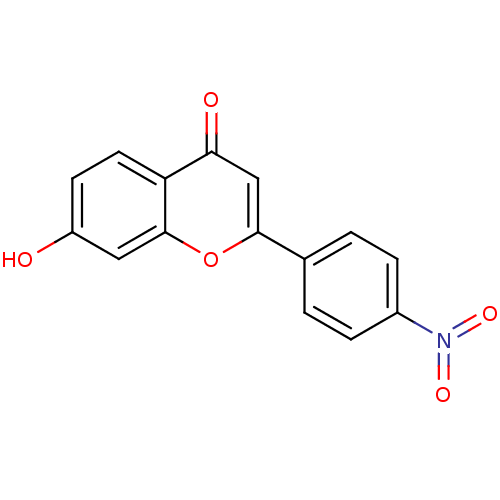
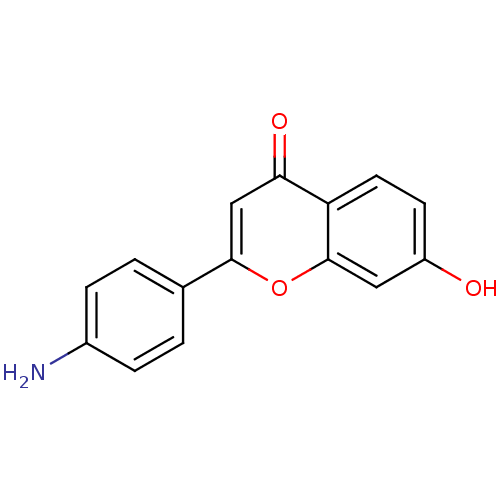
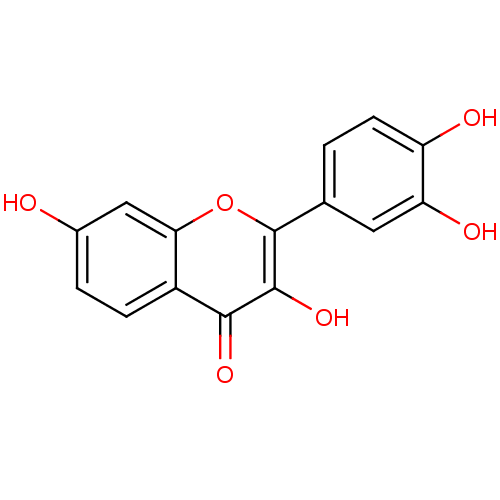
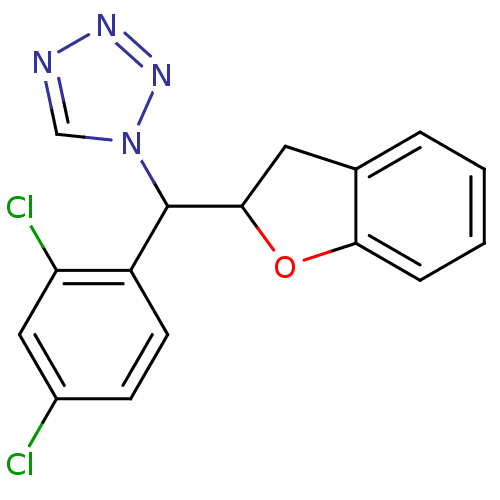
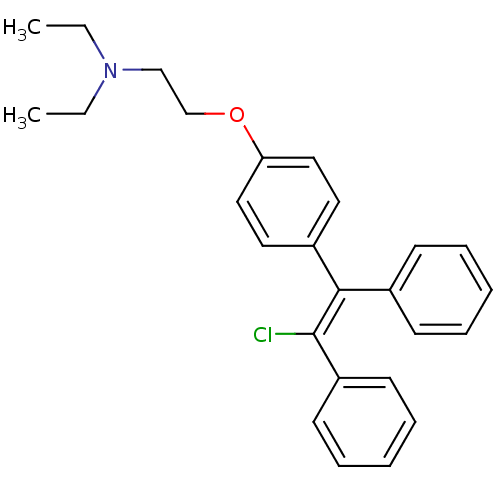
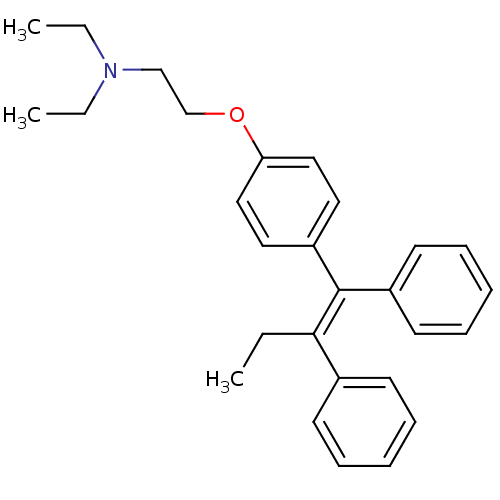
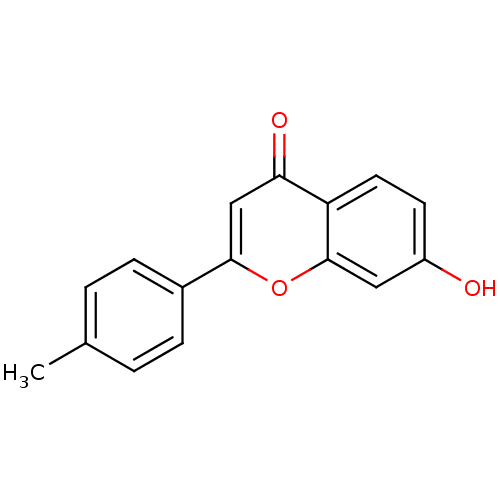
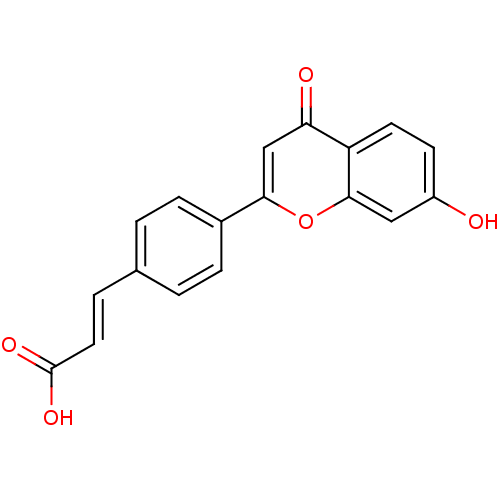
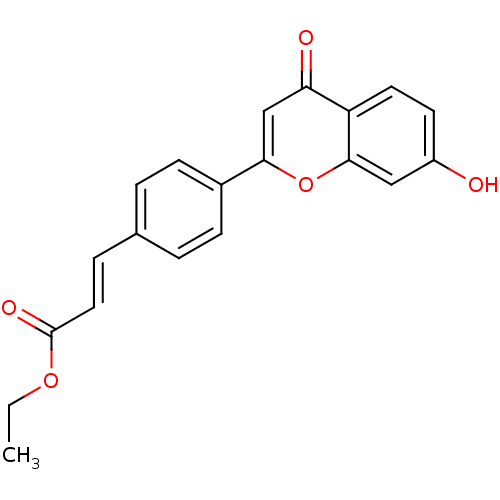
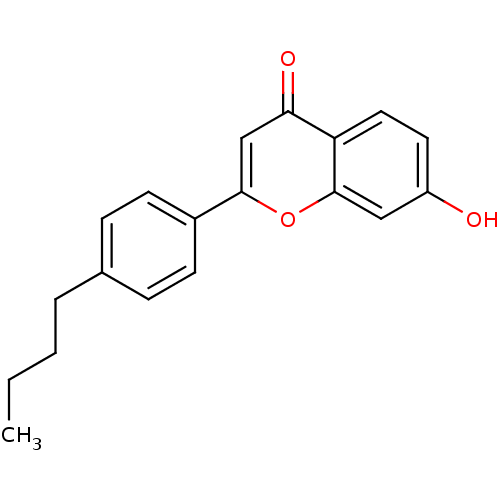
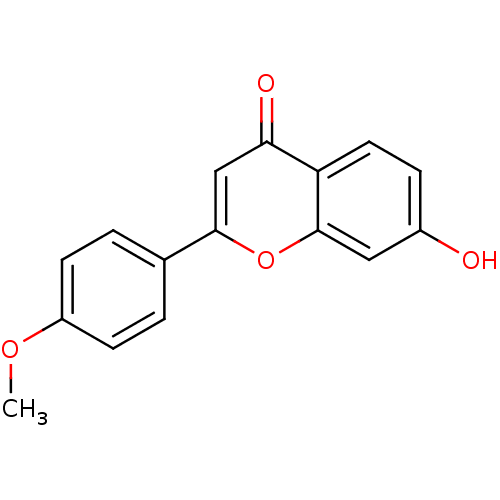
Affinity DataKi: 680nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataKi: 800nMAssay Description:Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM)More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataKi: 1.75E+3nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataKi: 1.75E+3nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 590nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 880nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 6.13E+3nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 8.30E+3nMAssay Description:Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 8.40E+3nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 9.00E+3nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 9.15E+3nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 9.30E+3nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.35E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.48E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 uM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.57E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.64E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 1.64E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 1.66E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against testosterone at 1.5 microM (Km=0.13 uM)More data for this Ligand-Target Pair
Affinity DataIC50: 1.72E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+4nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of Cytochrome P450 19A1 against Androstenedione at 0.25 uM (Km=55 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.13E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 3.03E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 3.24E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+4nMAssay Description:Inhibition of human placental aromatase, cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 7.62E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 9.81E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMT: 2°CAssay Description:The classical 3H2O assay was used to measure the effect of the flavones on aromatase activity using human placental microsomes.More data for this Ligand-Target Pair