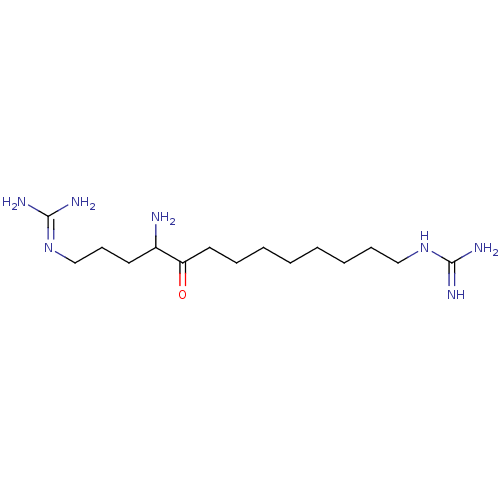
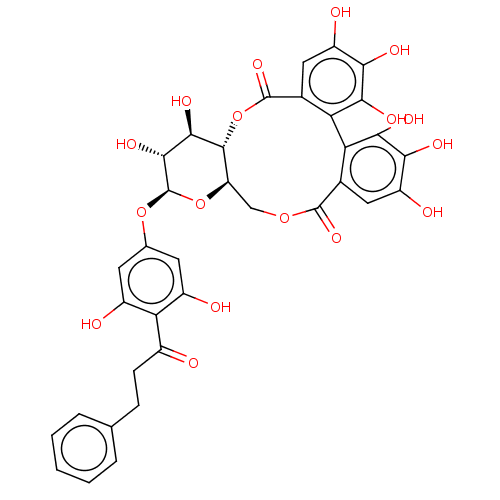
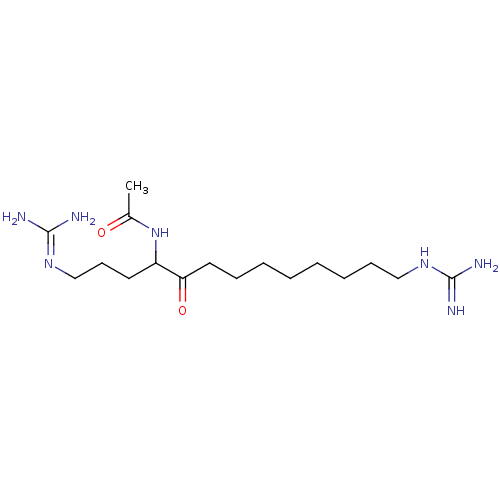
Affinity DataIC50: 6nMAssay Description:Displacement of QNB from muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Displacement of QNB from muscarinic M1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Displacement of QNB from muscarinic M4 receptorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
TargetCyclin-dependent kinase 1(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
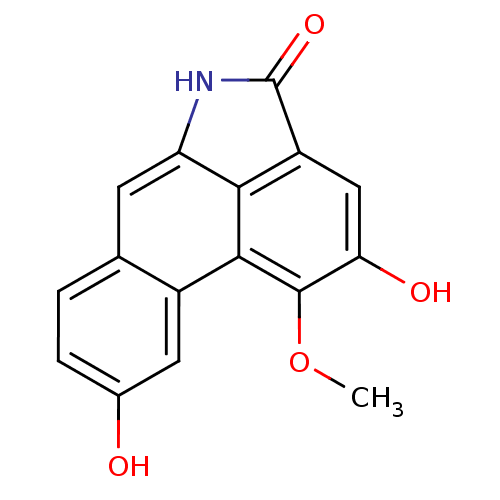
Affinity DataIC50: 300nMAssay Description:Inhibitory activity of the compound evaluated in the HCV NS3 protease activity assayMore data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
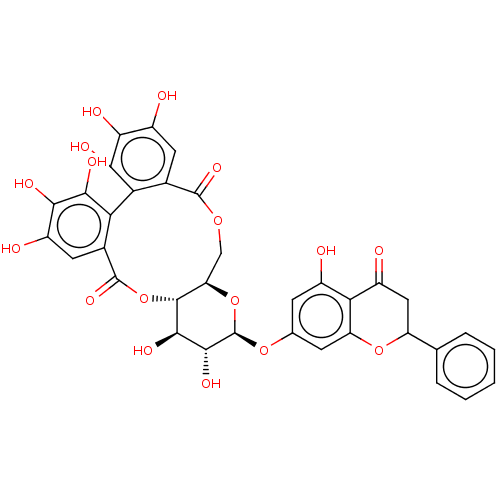
Affinity DataIC50: 300nMAssay Description:Compound was evaluated for inhibitory activity against cholesteryl ester transfer proteinMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibitory activity of the compound evaluated in the HCV NS3 protease assayMore data for this Ligand-Target Pair
Affinity DataIC50: 910nMAssay Description:Displacement of QNB from muscarinic M4 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 960nMAssay Description:Displacement of QNB from muscarinic M2 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.41E+3nMAssay Description:Displacement of QNB from muscarinic M1 receptorMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
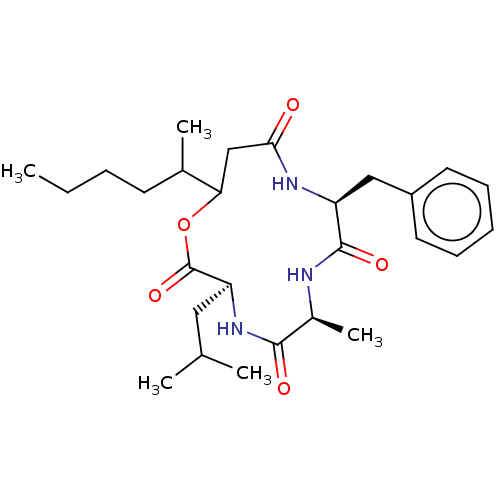
Affinity DataIC50: 1.42E+3nMAssay Description:Inhibition of CDK4More data for this Ligand-Target Pair
Affinity DataIC50: 2.14E+3nMAssay Description:Inhibition of Aurora 2 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibitory activity of the compound evaluated in the HCV protease binding assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.70E+3nMAssay Description:Inhibition of 50% of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CDK2More data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Tested for inhibition of substrate hydrolysis in the presence of porcine kidney esterase at a concentration of 45 nMMore data for this Ligand-Target Pair
TargetCholesteryl ester transfer protein(Homo sapiens (Human))
Schering Plough Research Institute
Curated by ChEMBL
Schering Plough Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.00E+4nMAssay Description:pA2 value towards endothelin receptor A was determined as functional ETA antagonismMore data for this Ligand-Target Pair