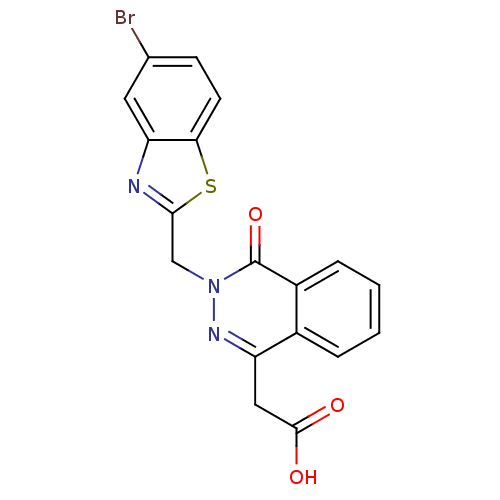
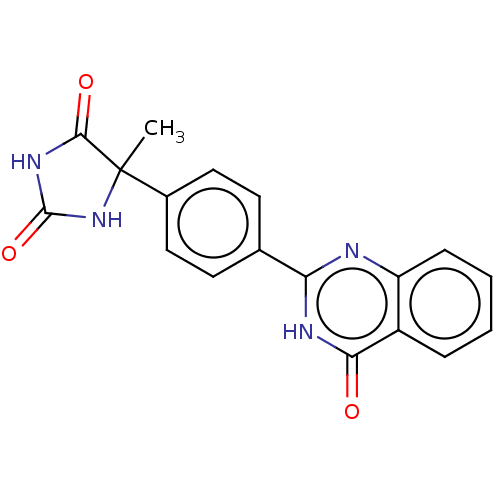
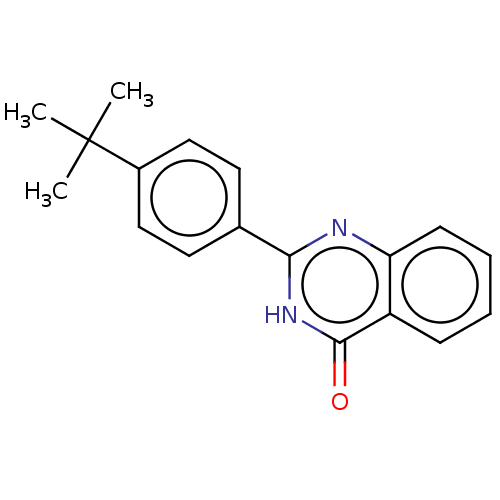
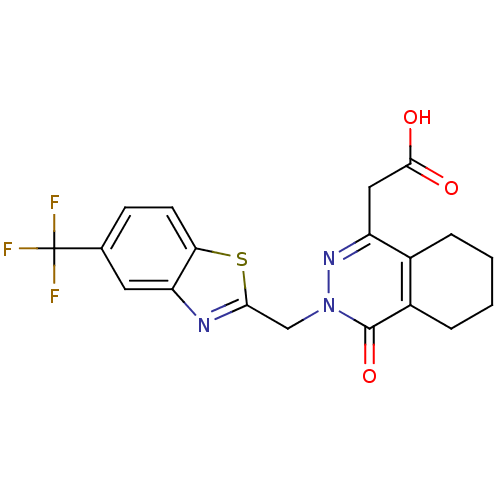
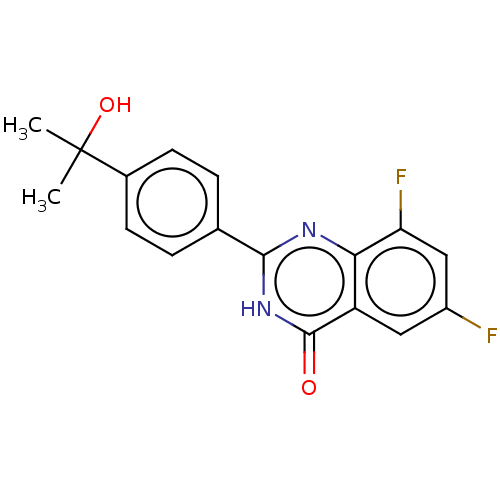
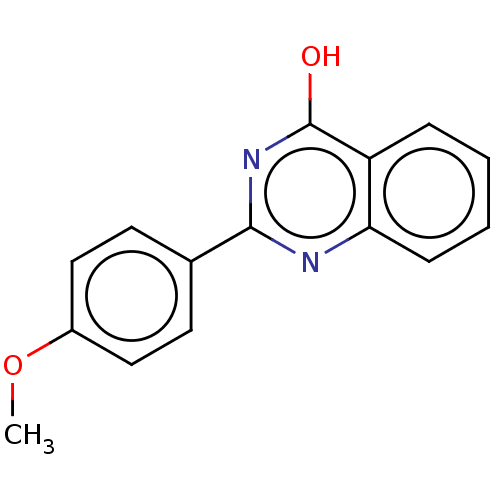
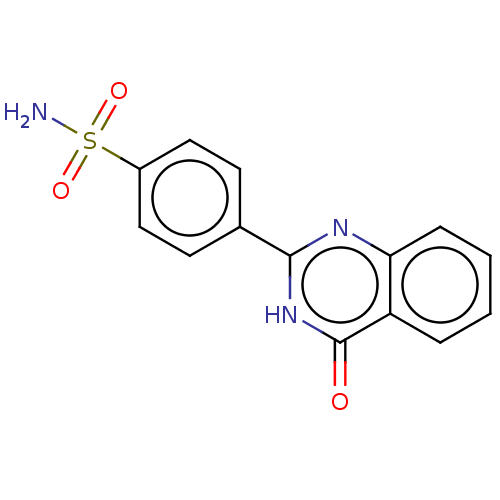
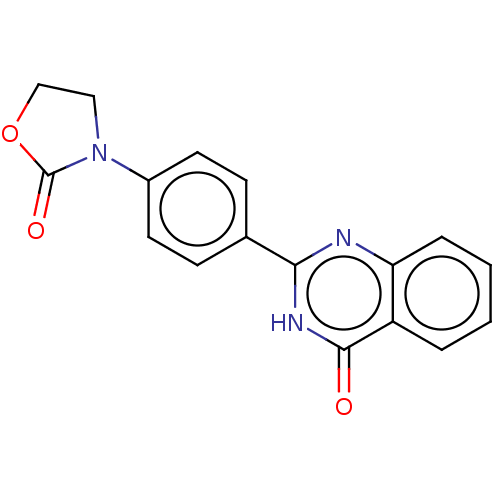
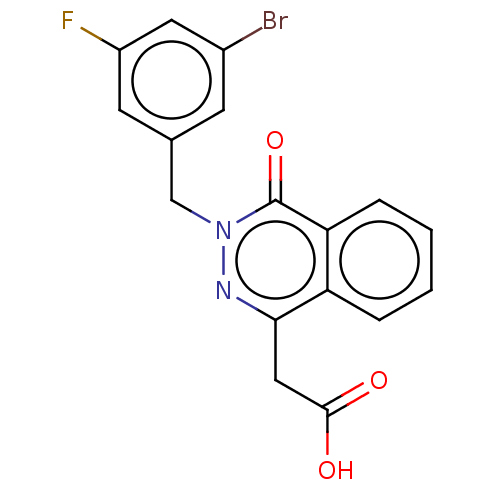
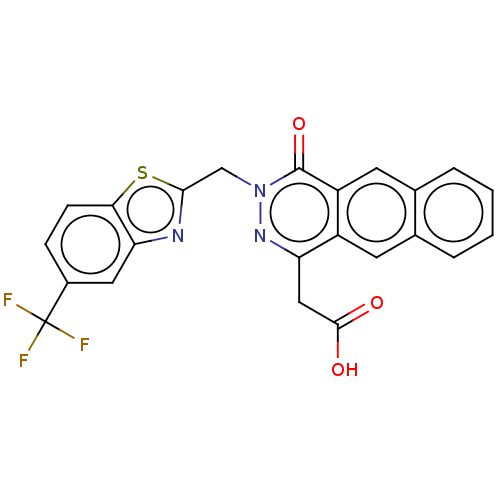
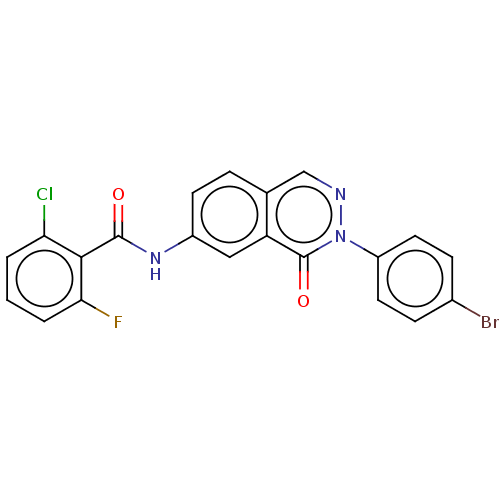
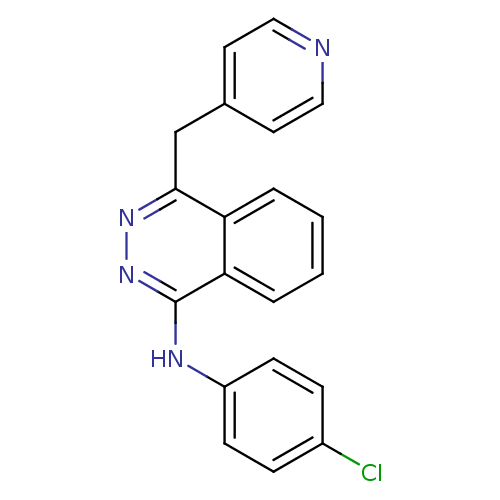
Affinity DataKi: 61nMAssay Description:Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric methodMore data for this Ligand-Target Pair
Affinity DataKi: 61nMAssay Description:Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric methodMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human PARP1 expressed in Escherichia coli incubated for 15 to 40 mins by ELISAMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human PARP1 expressed in Escherichia coli incubated for 15 to 40 mins by ELISAMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human PARP1 expressed in Escherichia coli using histone as substrate by ELISAMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 2(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of PARP2 (unknown origin)More data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 2(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 2.80nMAssay Description:Inhibition of PARP2 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 3.60nMAssay Description:Inhibition of PARP1 (unknown origin) by colorimetric assayMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 5.20nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 6.20nMAssay Description:Inhibition of PARP1 (unknown origin) by colorimetric assayMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 8.40nMAssay Description:Inhibition of human PARP1 expressed in Escherichia coli incubated for 10 mins by colorimetric assayMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 8.40nMAssay Description:Inhibition of human PARP1 expressed in Escherichia coli incubated for 10 mins by colorimetric assayMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
In DepthDetails
In DepthDetails
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of human placenta Aldose reductaseMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
In DepthDetails
In DepthDetails
TargetAldo-keto reductase family 1 member B1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 46nMAssay Description:Inhibition of human Aldose reductaseMore data for this Ligand-Target Pair
TargetProstaglandin E synthase(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of mPGES-1 in human A549 cells assessed as reduction in IL-beta induced PGE2 release preincubated for 30 mins followed by IL-beta addition...More data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of N-terminal GST-tagged human PARP1 expressed in a Baculovirus infected Sf9 insect cells using biotinylated substrate incubated for 1 hr ...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of VEGFR2 (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of VEGFR1 (unknown origin)More data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 139nMAssay Description:Inhibition of N-terminal GST-tagged human PARP1 expressed in a Baculovirus infected Sf9 insect cells using biotinylated substrate incubated for 1 hr ...More data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of C-terminal His4-tagged Aurora A (unknown origin) using PKB-GSK2 biotinylated peptide as substrate incubated for 90 mins by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 426nMAssay Description:Inhibition of Acetylcholinesterase (unknown origin) using acetylthiocholine iodide as substrate by Ellman's spectrophotometric methodMore data for this Ligand-Target Pair
TargetPoly [ADP-ribose] polymerase 1(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human PARP1 expressed in Escherichia coli incubated for 15 to 40 mins by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of ovine COX1 preincubated for 5 mins followed by arachidonic acid addition and measured after 2 mins by colorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.50E+3nMAssay Description:Inhibition of ovine COX1 preincubated for 5 mins followed by arachidonic acid addition and measured after 2 mins by colorimetric methodMore data for this Ligand-Target Pair
TargetCystic fibrosis transmembrane conductance regulator(Homo sapiens (Human))
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Y. B. Chavan College of Pharmacy
Curated by ChEMBL
Affinity DataEC50: <1.00E+4nMAssay Description:Inhibition of human CFTR F508 deletion mutant expressed in rat FRT cells coexpressing YFP-H148Q/I152L 25,22 incubated for 60 to 120 mins in presence ...More data for this Ligand-Target Pair

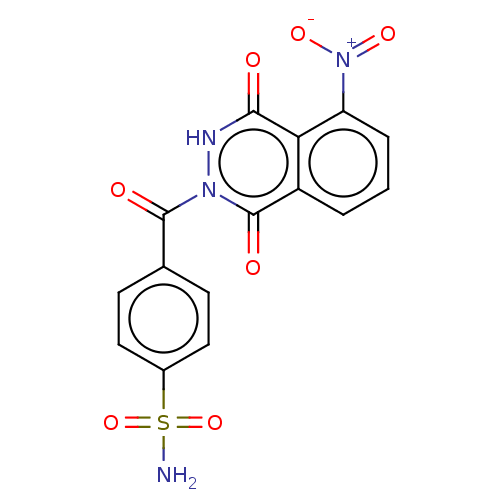




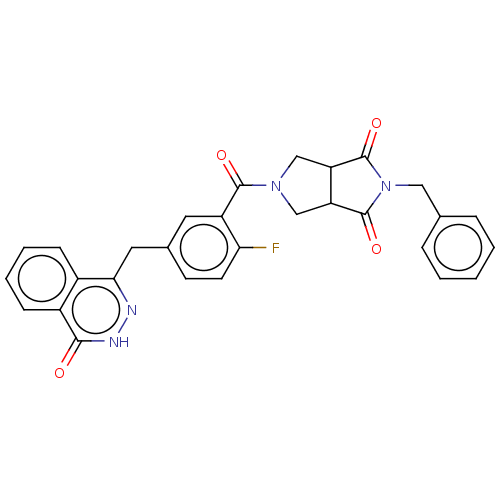



 3D Structure (crystal)
3D Structure (crystal)