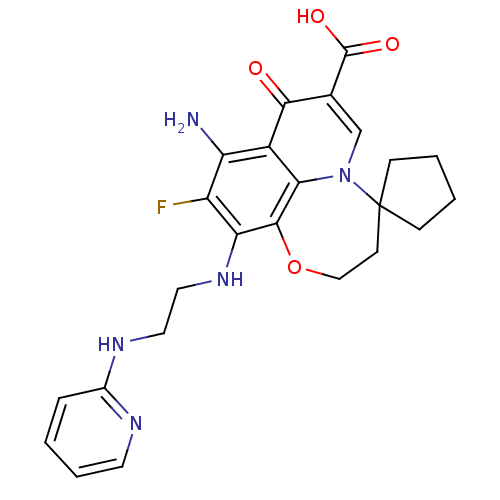
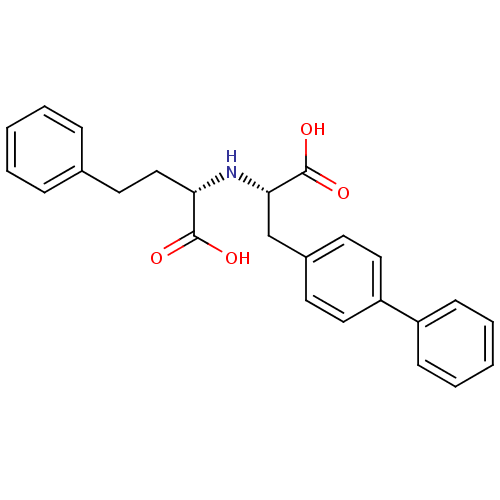
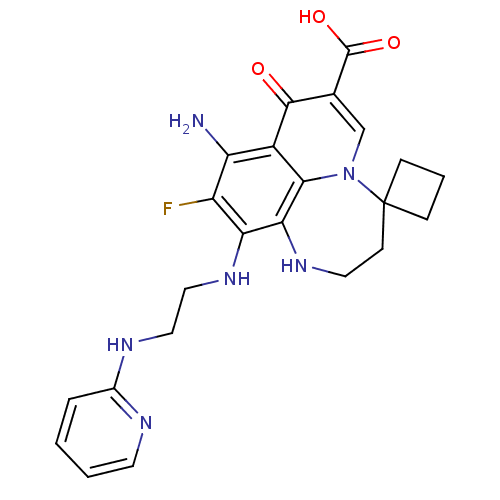
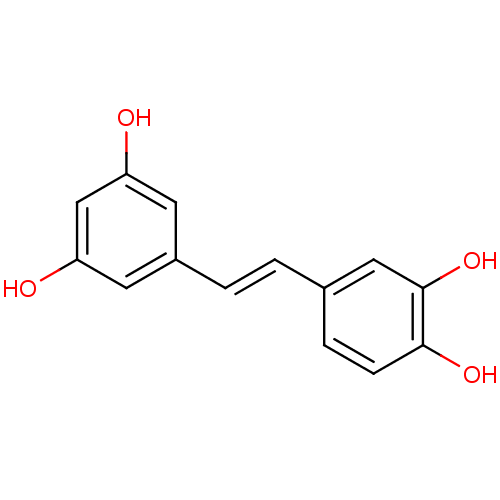
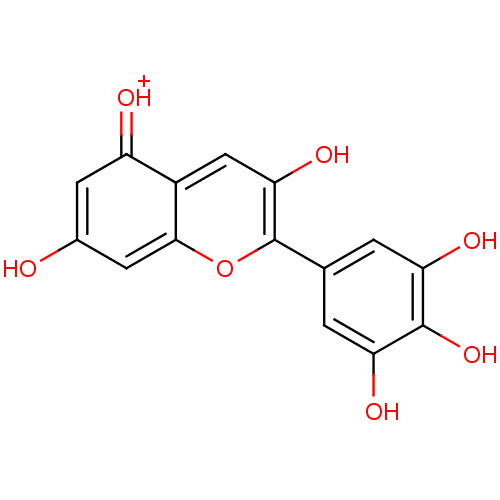
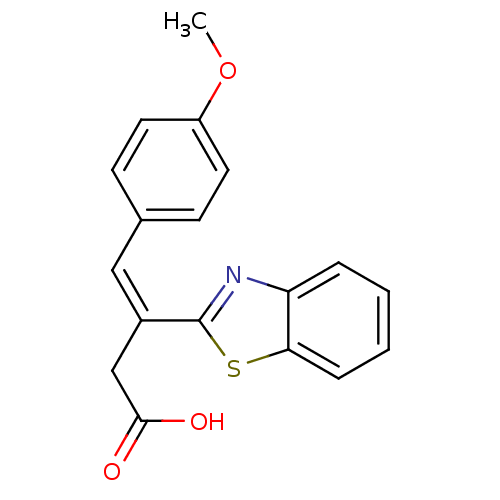
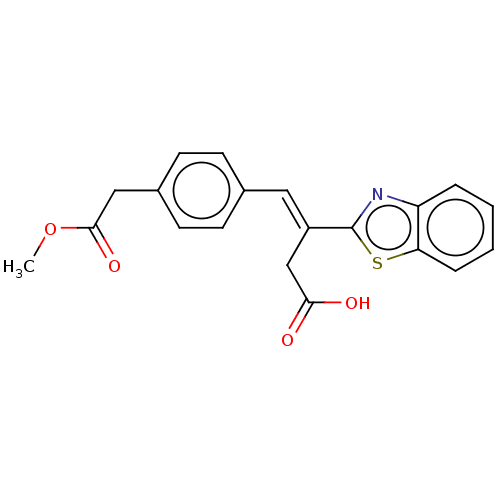
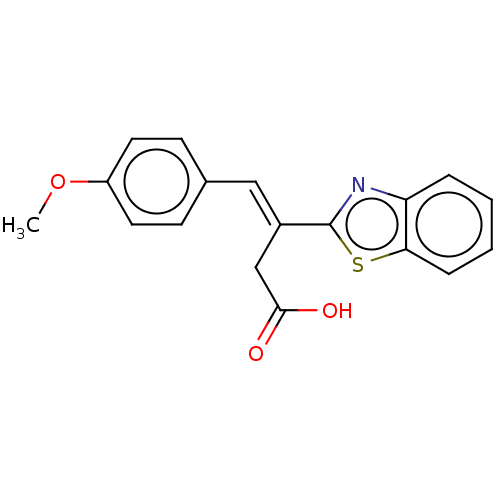
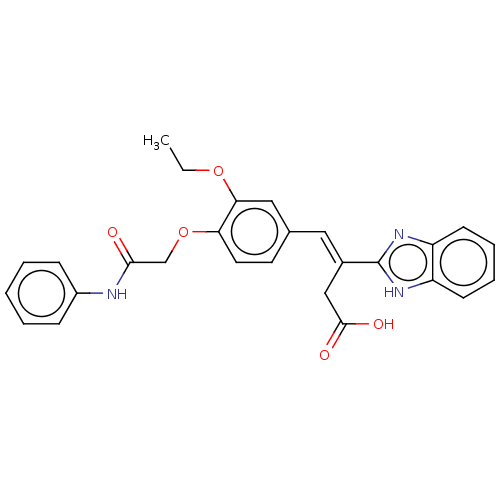
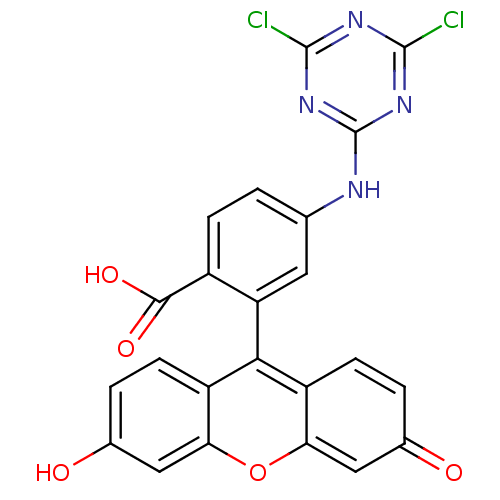
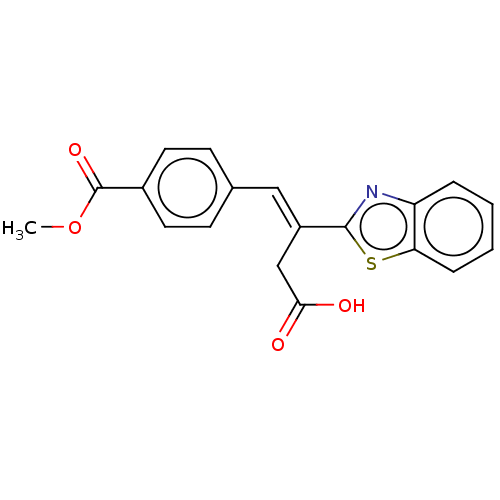
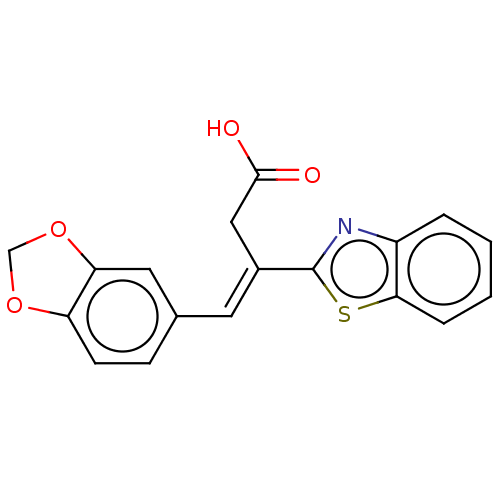
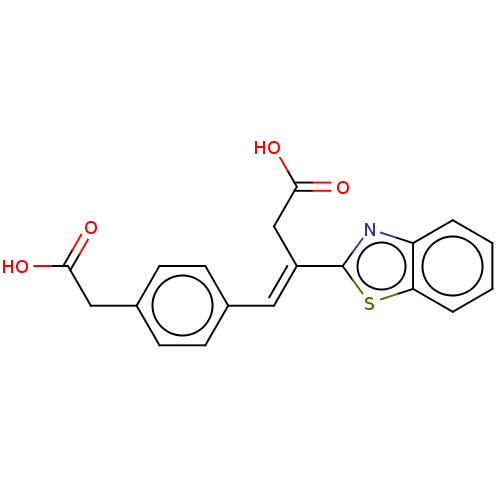
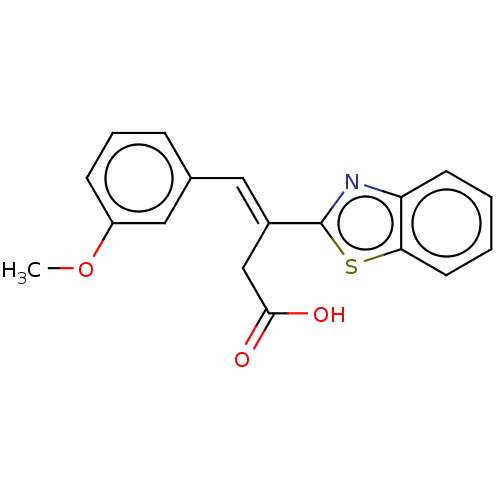
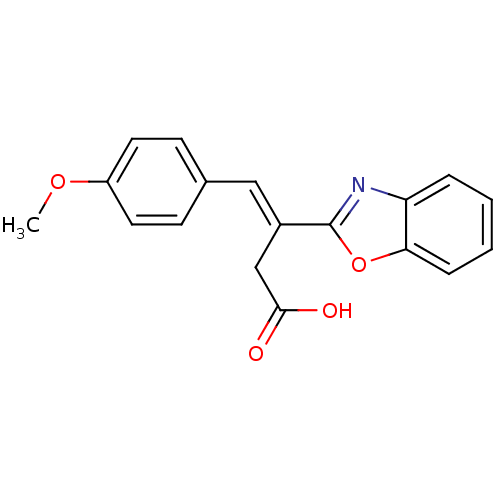
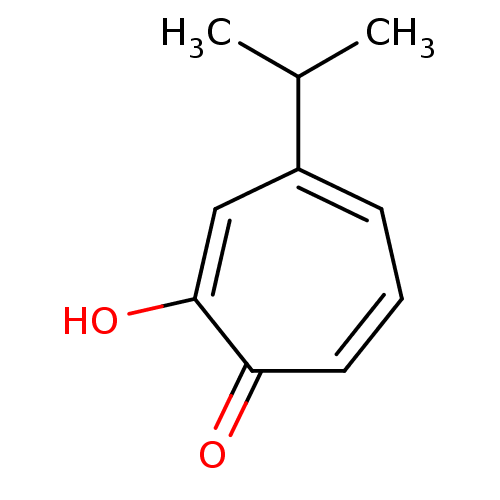
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysisMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysisMore data for this Ligand-Target Pair
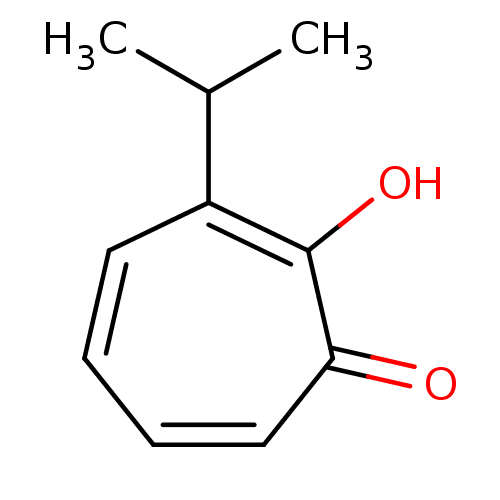
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Competitive inhibition of mushroom tyrosinase after 15 mins by Lineweaver-Bulk plot analysisMore data for this Ligand-Target Pair
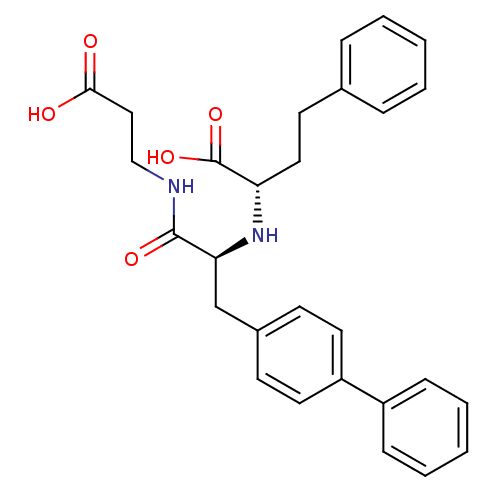
Affinity DataIC50: 0.360nMAssay Description:Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.510nMAssay Description:Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 3(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of Flt4 (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ...More data for this Ligand-Target Pair
Affinity DataIC50: 9.70nMAssay Description:Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ...More data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of KDR (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of GST-Flt1 kinase domain (unknown origin) expressed in baculovirus infected Sf9 cells after 20 mins by scintillation countingMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 30nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 36nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Affinity DataIC50: 70nMAssay Description:Inhibition of mushroom tyrosinase after 15 minsMore data for this Ligand-Target Pair
TargetPolyphenol oxidase 2(Agaricus bisporus (Common mushroom))
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Institute for Theoretical Medicine, Inc.
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of mushroom tyrosinase after 15 minsMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of PDGFR-alpha (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of PDGFR-beta (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 420nMAssay Description:Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ...More data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ...More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor beta(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 480nMAssay Description:Inhibition of PDGFR-beta (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 3(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 500nMAssay Description:Inhibition of Flt4 (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Kyorin Pharmaceutical Co., Ltd
Curated by ChEMBL
Affinity DataIC50: 550nMAssay Description:Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assayMore data for this Ligand-Target Pair
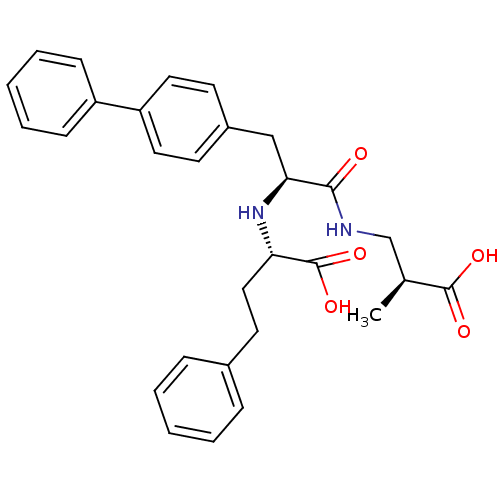
Affinity DataIC50: 560nMAssay Description:Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 760nMAssay Description:Inhibition of human N-terminal His6-tagged GLO1 expressed in baculovirus infected sf21 cells assessed as reduction in S-D-lactoylglutathione formatio...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of KDR (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human recombinant glyoxalase 1 assessed as S-D-lactoylglutathione after 15 mins by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of recombinant human His-tagged glyoxalase 1 expressed in Escherichia coli BL21 assessed as formation of S-D-lactoylglutathione after 5 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of EGFR (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 1(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of GST-Flt1 kinase domain (unknown origin) expressed in baculovirus infected Sf9 cells after 20 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of human recombinant His-tagged Glyoxalase 1 expressed in Sf21-Baculovirus systemMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of human recombinant DNase gamma expressed in Rosetta DE3 cells assessed as increase in acidsoluble DNA after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha(Homo sapiens (Human))
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Institute of Microbial Chemistry (BIKAKEN)
Curated by ChEMBL
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of PDGFR-alpha (unknown origin) after 20 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.60E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of Glyoxalase-1 (unknown origin) assessed as inhibition of S-D-lactoylglutathione formation using MG and reduced glutathione as substrate ...More data for this Ligand-Target Pair

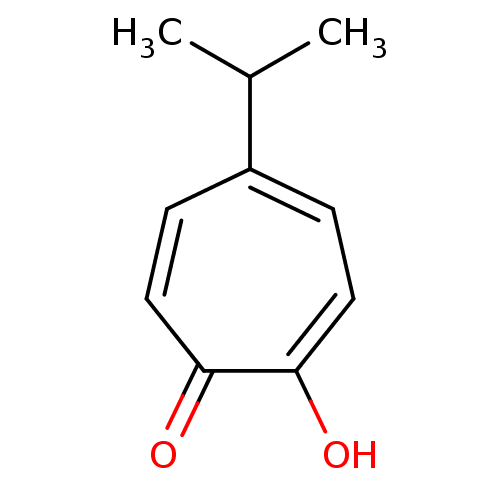
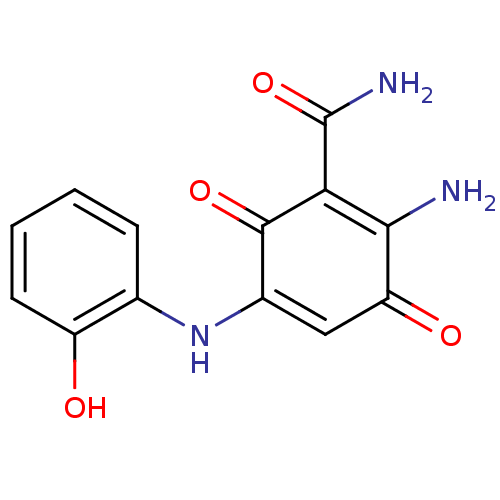

 3D Structure (crystal)
3D Structure (crystal)